Reversibility of adverse cardiac remodeling in type 2 diabetes mellitus patients: focus on sodium-glucose cotransporter-2 inhibitors
Abstract
Sodium-glucose cotransporter-2 (SGLT2) inhibitors have been recently approved by world-reputed medical associations as a milestone of class A management of heart failure (HF) with reduced ejection fraction (HFrEF) after pooling strong evidence (mainly for dapagliflozin or empagliflozin) regarding their beneficial impact on total occurrences of cardiovascular deaths and hospitalizations for HF in patients with and without type 2 diabetes mellitus (T2DM). Having a wide range of profile of favorable pleiotropic effects on heart, vessels, and kidney, SGLT2 inhibitors probably have a class-specific tissue protective ability, while its exact molecular mechanism has not been clearly understood yet. However, whether these agents retain their potency to reverse adverse cardiac remodeling remains unclear. The review elucidates the role of SGLT2 inhibitors in the potential reversibility of cardiac remodeling in connection with the improvement of clinical outcomes among T2DM patients having HF. Herein, we discussed the effects of SGLT2 inhibitors on cardiac structure and hemodynamics in T2DM patients. We revealed that empagliflozin had sufficient benefits in alleviating the adverse cardiac remodeling in HFrEF individuals than other SGLT2 inhibitors. These findings can open a new vision for the optimization of HF therapy in the near future.
Keywords
INTRODUCTION
Adverse cardiac remodeling is a general characteristic of heart structural abnormalities and impaired diastolic and systolic functions, which reflect a natural evolution of a large number of cardiovascular (CV) diseases, such as acute coronary syndrome or acute myocardial infarction, valvular heart disease, cardiomyopathies, and myocarditis[1]. The pathogenesis of adverse cardiac remodeling (ACR) is complex, and it is strongly related to heart failure (HF) development and progression[2,3]. Indeed, changes in the structure of the left ventricle (LV) at the different stages of HF development involve multiple interactions between myocardial cellular components and extracellular matrix, which are under tight auto-paracrine regulation[4]. Ischemia/reperfusion, atherosclerosis, and microvascular obstruction, along with conventional CV risk factors (e.g., hypertension, dyslipidemia, and smoking), as well as metabolic co-morbidities, such as type 2 diabetes mellitus (T2DM), insulin resistance, and overweight and obesity, are considered to be powerful triggers for adverse cardiac remodeling through activation of numerous cellular signaling pathways (e.g., calcineurin and calcium-/calmodulin-dependent protein kinases, nitric oxide–cyclic GMP-cascade, protein kinase G type Iα activity, and phosphatidylinositol 3-kinase/Akt/mTOR signaling) directly and indirectly influencing viability of myocardium and modification of its architecture[5,6]. Finally, mitochondrial dysfunction, oxidative stress, autophagy, and apoptosis lead to progressive cardiac myocyte loss, cardiac hypertrophy and extensive interstitial fibrosis[7,8]. Cumulatively, these changes intervene in transitioning from adaptive cardiac remodeling into maladaptive ACR, reflecting an occurrence of diastolic and systolic myocardial dysfunction[9]. The presence of enlarged cardiac cavities, LV hypertrophy, global strain rate alteration, and reduced LV ejection fraction (LVEF) are consistently associated with the advance of HF and poor clinical outcomes[10].
During the last decades, ACR has been regarded to be a target for point-of-care in patients with HF. However, the implementation of a four-pillar scheme [i.e., sodium-glucose cotransporter 2 (SGLT2) inhibitor, angiotensin receptor-neprilysin inhibitor along with beta-blockers, and mineralocorticoid receptor antagonist] in the management of HF led to undoubted benefits in a reversion of ACR[11]. Even though SGLT2 inhibitors have exerted a sufficient potency to improve the untoward course of T2DM decreasing all-cause and CV mortality, admission due to HF development, the underlying molecular mechanisms of reversibility of ACR, remains poorly understood. Moreover, recent studies have demonstrated that SGLT2 inhibitors exerted their additional CV effects in close dependence on concomitant medication. Indeed, Patoulias et al. (2021)[12] pooled the findings from the three most important randomized placebo-controlled clinical trials (RCTs) including DAPA-HF, EMPEROR-Reduced, and VERTIS CV, with a total of 16,720 T2DM patients. They revealed that SGLT2 inhibitors demonstrated their additional CV benefits in close relation to concomitant medications (composite of total HF admission + CV death). For instance, among HF with reduced ejection fraction (HFrEF), patients receiving mineralocorticoid antagonists or angiotensin receptor-neprilysin inhibitors, treatment with SGLT-2 inhibitors resulted in a remarkable decrease in the primary outcome, while among those not receiving loop diuretics, SGLT-2 inhibitors were not superior to placebo. Despite the fact that different phenotypes of HF [i.e., HFrEF (EF < 40%), HF with mildly reduced (HFmrEF, EF = 40%-49%), and preserved (HFpEF, EF ≥ 50%) ejection fraction] are sufficiently distinguished in etiology, age/gender predominance, and signature of co-morbidities and coexisting conditions, SGLT2 inhibitors exerted their positive impact in the improvement of CV mortality and reduction of HF readmission regardless on HF. As a result, the United States Food and Drug Association and the European Medical Agency have recently approved SGLT2 inhibitors (mainly empagliflozin) for use in HF regardless of their phenotypes[13,14], but it remained unclear whether SGLT2 inhibitors benefit outcomes in HF patients in connection with a reversion of ACR[15]. The aim of the review is to elucidate the role of SGLT2 inhibitors in the potential reversibility of ACR through attenuation of the untoward clinical course of disease in T2DM patients.
METHODS AND METHODOLOGY
We searched MEDLINE, EMBASE, Medline (PubMed), the Web of Science, and the Cochrane Central for English written articles with the following key words: [adverse cardiac remodeling], [heart failure], [HFrEF], [HFmrEF], [HFpEF], [diabetes mellitus], [type 2 diabetes mellitus], [cardiovascular risk], [sodium-glucose cotransporter-2 inhibitors], [empagliflozin], [dapagliflozin], [canagliflozin], and [ertugliflozin]. All the authors independently selected original articles, evaluated their quality, possible bias, presentation, and interpretation in correspondence to the study purpose, and shaped the final reference list.
SGLT2 INHIBITORS
Plausible mechanisms of actions
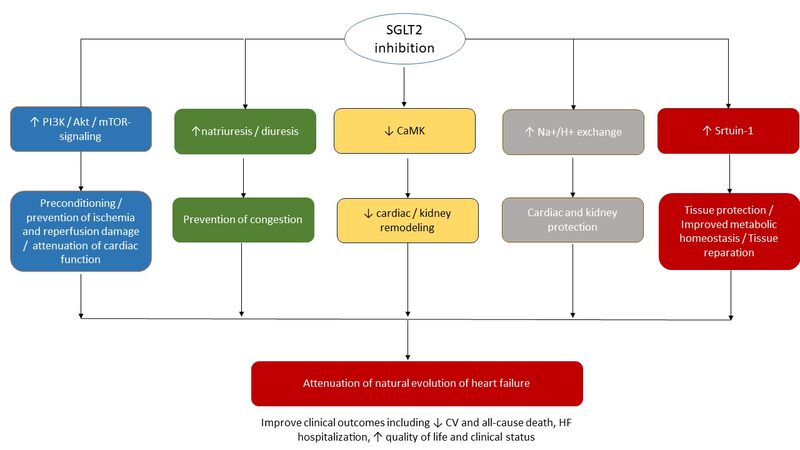
SGLT-2 inhibitors were originally designed as anti-diabetic agents with a unique molecular mechanism that affects the regulation of SGLT-2-related glucose reabsorption in proximal tubule of nephron resulting in glycosuria, decreasing fasting plasma glucose, reducing glycated hemoglobin (HbA1c), and resulting in weight loss. However, a wide range of the effects of SGLT2 inhibitors with serious clinical significance has been found[16]. Nevertheless, mild-to-moderate hypoglycemic effect of these agents was not closely corresponded to their dramatic favorable pleiotropic effects on CV and renal outcomes, which requires an additional explanation of their mechanism of actions. The simplest assumption is considered to be realistic; a decrease in sustained systemic blood pressure results in natriuresis and declining activity of the sympathetic nervous system, which can translate into improvement of CV prognosis and slowing kidney disease progression. Body weight reduction may also be a potential mechanism for the alleviation of CV risk[17]. Apart from this, it has been hypothesized that SGLT2 inhibitors can be involved in the regulation of Na+/H+ exchange in heart and kidney, leading to both cardiac and renal protection. Through their stimulating effect on diuresis and natriuresis, these agents can decrease the interstitial osmotic gradient, pre-load, and after-load, and thereby potentially improve vascular structure and function[18]. Acting as stimulators of erythropoiesis due to “mimicking” effect of systemic hypoxia in the kidney, SGLT2 inhibitors seem to be powerful triggers for non-specific tissue protection[19,20]. In addition, they may modulate the production of a wide spectrum of adipokines (e.g., leptin, visfatin, and adiponectin), myokines (e.g., apelin and irisin) and pro-inflammatory cytokines (e.g., interleukin-2, interleukin-6, interferon-gamma, and tumor necrosis factor-alpha) through the impact of the sirtuin pathway[21]. This signaling pathway is also responsible for the turnover of myocardial metabolism from aerobic glycolysis to oxidation of other substrates, such as ketone acids, free fatty acids, and branched-chain amino acids, which appear to be a powerful modulator for mitochondrial function, playing a pivotal role in preconditioning and oxidative stress[22]. Finally, the sirtuin-1 pathway seems to be a central player in SGLT2-related regulation of reducing cardiac cells necrosis as well as cardiac and kidney fibrosis[22]. Figure 1 illustrates potent molecular mechanisms underlying tissue protective ability of SGLT2 inhibitors.
Figure 1. Underlying pathogenetic mechanisms of tissue protective effects of SGLT2 inhibitors↑. Akt: Serine/threonine-specific protein kinase; HF: heart failure; CaMK: Ca2+/calmodulin-dependent protein kinase; mTOR: mammalian target of rapamycin; SGLT2: sodium-glucose cotransporter 2; PI3K: phosphatidylinositol 3-kinase; ↑: increase; ↓: decrease.
Although there is much scientific evidence and assumptions about the effect of SGLT2 inhibitors on cardiac and kidney protection, the exact underlying molecular mechanisms of actions of these agents remain uncertain.
Cardiovascular and renal protective benefits of SGLT2 inhibitors in T2DM patients
Renal and myocardial benefits of SGLT2 inhibitors have been clearly documented in numerous observational and clinical studies[23]. The basic characteristics of the SGLT2 inhibitors from RCTs are reported in Table 1. A systematic review of 10 RCTs based on the data received from patients with T2DM and chronic kidney disease (CKD) with an estimated glomerular filtration rate (eGFR) > 30 mL/min/1.73 m2 has undeniably revealed that several SGLT2 inhibitors (empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin) were by far more effective than placebo in decreasing serum levels of fasting glucose and HbA1c[24]. Therefore, a reduced incidence of progressive declining eGFR slope on follow-up was noticed shortly after initiating SGLT2 inhibitors. In addition, SGLT2 inhibitors significantly reduced albuminuria and its turnover to proteinuria. Finally, another systematic review and meta-analysis of four RCTs (EMPA-REG OUTCOME, CANVAS Program, CREDENCE, and DECLARE-TIMI 58) depicted CV or renal outcome of SGLT2 inhibitors (empagliflozin, canagliflozin, and dapagliflozin); and based on the pooled data of a total of 38,723 participants, the study has shown the superiority of these agents versus placebo in a composite renal outcome (i.e., renal replacement therapy by dialysis, kidney transplantation, CKD-related death, end-stage CKD, and acute kidney injury) [25]. The meta-analysis of 27 RCTs, in which 7363 participants with T2DM and CKD were recruited, has convincingly demonstrated that SGLT2 inhibitors attenuated CV mortality risk mainly related to a decrease in non-fatal acute myocardial infarction, non-fatal stroke, or incident HF, whereas all-cause mortality rate was not remarkably reduced[26].
Most valued randomized clinical trials depicted SGLT2 inhibitors in type 2 diabetes mellitus and heart failure
| Acronym | Treatment | Patients | n | End points | Results |
| EMPA-REG outcome | Empagliflozin 10 mg vs. placebo | T2DM and atherosclerotic CV disease 65% had a prior MI or stroke | 7020 | CV death, all-cause mortality, 3-point MACE, and HF hospitalization | ↓ the risk of MACE, MI, coronary revascularization, and all-cause admission to hospital |
| CANVAS Program | 300 mg canagliflozin, 100 mg canagliflozin, or placebo | T2DM at risk of CV events | 10 142 | MACEs, renal outcomes | ↓ the risk of eGFR declining, ↓ albuminuria |
| CREDENCE | Canagliflozin 100 mg vs. placebo | T2DM with eGFR of 30 to < 90 ml/min per 1.73 m2 and substantial albuminuria | 4401 | Renal and CV outcomes in connection with the effects on eGFR slope | ↓ the risk of renal and CV events |
| DECLARE-TIMI 58 | Dapagliflozin 100 mg vs. placebo | T2DM with HbA1c 6.5%-12.0%, with either established atherosclerotic CV disease or multiple risk factors, and creatinine clearance of at least 60 mL/min | 17 160 | Cardiorenal composite outcome or death | ↓ the risk of end-stage CKD or renal death, CV mortality |
| DAPA-HF | Dapagliflozin 10 mg vs. placebo | stable HFrEF with or without T2DM | 4744 | Admission or an urgent visit for HF or CV death | ↓ CV mortality and worsening HF, improved signs and symptoms, ↑ physical status and ↑ quality of life |
| EMPEROR-Preserved | Empagliflozin 10 mg vs. placebo | stable HFpEF (LVEF > 40%) with or without T2DM | 5750 | CV mortality or HF hospitalization | ↓ the combined risk of death due to CV events, HF hospitalization, or an emergency or urgent HF admission requiring intravenous treatment, intensive care, and a vasopressor or positive inotropic drug use |
| EMPEROR-Reduced | Empagliflozin 10 mg vs. placebo | stable HFrEF (LVEF ≤ 40%) with or without T2DM | 3730 | Primary outcome (death due to CV events or HF admission), total hospitalizations for HF, and adverse renal outcomes | ↓ the risk of primary outcome and HF admission regardless of T2DM presence |
| SOLOIST-WHF | Sotagliflozin 200 mg vs. placebo | T2DM with HFrEF or HFpEF after a recent hospitalization for worsening HF | 1222 | HР hospitalizations | ↑ the number of days without hospital admission |
Zou et al. (2019)[27] polled the data of 61,076 patients with T2DM, who were selected in 42 clinical studies and treated with SGLT2 inhibitors or placebo. The authors have reported that the treatment with SGLT2 inhibitors was strongly associated with a significant reduction in the incidence of major adverse CV events (MACEs), acute myocardial infarctions, and CV and all-cause mortality. However, the risk of ischemic stroke was not reduced.
The meta-analysis of DAPA-HF (dapagliflozin) and EMPEROR-Reduced (empagliflozin) trials, which have been recently completed, has yielded strong evidence for the superiority of SGLT2 inhibitors versus placebo in reduction of all-cause mortality, death due to CV events, the combined risk of CV death and first HF hospitalization, the composite end point of recurrent HF admissions or CV death, and the risk of untoward renal outcomes in HFrEF patients with stable hemodynamics[28]. Yet, dapagliflozin and empagliflozin alone exhibited their favorable profile in significantly reducing the risk of HF decompensation, MACEs or death due to CV events when compared to placebo in HFrEF patients regardless of the presence of T2DM[29,30]. Principally to note, DAPA-HF and EMPEROR-Reduced have been mainly included hemodynamically stable patients with HFrEF (LVEF < 40%). Unlike the trials mentioned above, the SOLOIST-WHF trial selected patients shortly after urgent hospitalization for worsening HF who received a minimum of one dose of innovative SGLT1/2 inhibitor sotagliflozin or placebo prior to the discharge (48.8%) or within two days after discharge (51.2%). The primary combined end point in the study was referred to as CV deaths, HF hospitalizations, and urgent visits for HF manifestation[31]. The authors found that the total number of pre-specified clinical outcomes occurred sufficiently lower in the sotagliflosin group than in the placebo group[31]. In addition to that, empagliflozin in the EMPEROR-Preserved trial appeared to be the first agent from the SGLT2 inhibitors that unequivocally reduced the risk of CV morbidity or admission due to HF in HFpEF patients with and without T2DM[32]. Thus, SGLT2 inhibitors (predominantly empagliflozin and dapagliflozin) have demonstrated their ability to improve survival and prevent HF hospitalization mainly in HFrEF, whereas their potency to reduce mortality did not clearly relate to attenuation of ACR. In addition, SGLT2 inhibitors (empagliflozin, dapagliflozin, canagliflozin, ertugliflozin, and luseogliflozin) improved renal outcomes and declined the risk of HF occurrence in T2DM patients.
Meta-analyses of clinical studies devoted to SGLT2 inhibitors’ effect on adverse cardiac remodeling
The meta-analyses, which have been previously reported, have demonstrated controversial data regarding cardiac protection and the possibility to reverse adverse cardiac remodeling. Indeed, Yu et al. (2021)[33] did not find strong evidence that SGLT2 inhibitors influence ACR parameters, such as LV mass index, LV end-diastolic and end-systolic volume indexes, or left atrial volume index; however, the beneficial changes in the E/e' ratio, biological markers (e.g., NT-pro brain natriuretic peptide) and quality of life have been concisely determined. In addition, SGLT2 inhibitors appeared to be significantly effective in LV ejection fraction increase solely among patients with HFrEF. The authors concluded that the use of SGLT2 inhibitors in T2DM patients regardless of HF was closely associated with substantial improvement of diastolic function, but not with structural parameters of adverse cardiac remodeling. In another meta-analysis that has been recently published by Dhingra et al. (2021)[34], five randomized clinical trials (n = 408) have been included with the aim of comparing the effects of SGLT2 inhibitors and placebo (the period of treatment > 3 months) on structural cardiac parameters reflecting ACR, which was determined by cardiac magnetic resonance imaging. Authors found that management of HF with SGLT2 inhibitors was associated with a more remarkable regression of LV mass but not LV mass index, than in the placebo group, while there was no significant difference in these parameters depending on HF phenotype.
The meta-analysis of 13 RCTs by Zhang et al. (2021)[35] has evaluated the impact of four SGLT2 inhibitors (i.e., empagliflozin, canagliflozin, dapagliflozin, and luseogliflozin) on cardiac performances that concisely characterize ACR in T2DM patients with or without known HF. Although ACR was not in the focus of assessment in most of the studies, four of them (i.e., SUGAR-DM-HF, EMPA-TROPISM, REFORM, and DAPACARD) had pre-specified magnetic resonance imaging for the main study or echocardiographic for the sub-study[36-39]. In fact, other studies that were included in the meta-analysis focused on both clinical courses of the disease and imaging features of ACR. To up-to-date knowledge, the methodological approaches of the meta-analysis did not exert an impact on its final interpretation. The overall population was composed of 1251. T2DM patients managed with SGLT2 inhibitors. The authors reported that conventional characteristics of ACR, including LVEF, LV mass, LV mass index, dimensions and volumes of left cavities, and E-wave deceleration time, were beneficially improved during the therapy. In addition to that, the assessment of cardiac images in subgroups yielded proof of the protective effects of SGLT2 inhibitors on ACR in HF patients independently from their glycemic status. In addition, the most profound reversion of ACR was confirmed in HFrEF patients mainly treated with empagliflozin than those with other SGLT2 inhibitors. The authors, thus, reported that the reversibility of ACR in HFrEF patients is considered to be a remarkable attribute of the innate capabilities of empagliflozin, which is considered to be more beneficial than other SGLT2 inhibitors regardless of glycemic status. Admittedly, these findings are regarded to be remarkably impressive for HF management because they open new dawn for HF patients. The current European Cardiology Society clinical guideline has also included new indications for SGLT2 inhibitors, which are now emphasized as: (1) reduction of the risk of HF hospitalization and death for T2DM patients at higher risk of HF; and (2) improving a clinical status along with reducing the risk of HF hospitalization and CV mortality in HFrEF patients regardless of their glycemic status[38-40]. However, only two SGLT2 inhibitors (dapagliflozin or empagliflozin) are seemed to be recommended for HFrEF patients, while significantly more molecules of these agents have been incorporated into the recommendations for the medical care of T2DM patients. In fact, the greatest weakness of the finding is that there is a lack of direct comparisons between different agents to prevent misunderstanding in possible class-specific effects received in RTCs. The meta-analysis provided a remarkable confirmation of the unequal efficacy of SGLT2 inhibitors on a reversion of ACR in HFrEF patients, which is related to the finding that empagliflozin appeared to be superior to other drugs from the group.
Last but not least, SGLT2 inhibitors are considered to be a quite intriguing clinical approach to speculate whether there are able to restore global cardiac function and attenuate ACR in connection with declining unfavorable outcomes in de novo acute and chronic HF in post-ST elevation myocardial infarction individuals or patients with acute myocarditis[41].
CONCLUSION
Favorable pleiotropic effects of SGLT2 inhibitors on clinical outcomes and cardiac and kidney function have been more profoundly established in T2DM patients regardless of HF phenotype presentation and in individuals with prevalent HF, mostly with HFrEF independently from T2DM. The results of recent meta-analyses have shown that at least four SGLT2 inhibitors (empagliflozin, dapagliflozin, canagliflozin, and luseogliflozin) demonstrated their superiority over placebo on a reversion of ACR in T2DM patients with and without HF. Along with it, it remains uncertain whether these agents yield a class-related effect on cardiac structure and function, quality of life, well-being, and clinical course. Future large clinical trials need to be provided with the aim of elucidating the plausible impact of different SGLT2 inhibitors on adverse cardiac remodeling in numerous patient populations using direct face-to-face comparison.
DECLARATIONS
Authors’ contributionEqually responsible for the conception, design, methodology, data pooling and their interpretation as well as in final approval of the proof of the paper: Berezin AE, Berezin AA
Availability of Data and MaterialsNot applicable.
Financial Support and SponsorshipNone.
Conflict of interestAll authors declared that there are no conflicts of interest.
Ethical Approval and Consent to ParticipateNot applicable.
Consent for PublicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Legallois D, Hodzic A, Alexandre J, et al. Definition of left ventricular remodelling following ST-elevation myocardial infarction: a systematic review of cardiac magnetic resonance studies in the past decade. Heart Fail Rev 2022;27:37-48.
2. Yang D, Liu HQ, Liu FY, et al. The Roles of noncardiomyocytes in cardiac remodeling. Int J Biol Sci 2020;16:2414-29.
3. Berezin AE, Berezin AA. Adverse cardiac remodelling after acute myocardial infarction: old and new biomarkers. Dis Markers 2020;2020:1215802.
4. Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep 2017;19:71.
5. Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol 2015;89:1401-38.
7. Schirone L, Forte M, Palmerio S, et al. A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxid Med Cell Longev 2017;2017:3920195.
8. Wu QQ, Xiao Y, Yuan Y, et al. Mechanisms contributing to cardiac remodelling. Clin Sci (Lond) 2017;131:2319-45.
9. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Journal of the American College of Cardiology 2000;35:569-82.
10. Reindl M, Reinstadler SJ, Tiller C, et al. Prognosis-based definition of left ventricular remodeling after ST-elevation myocardial infarction. Eur Radiol 2019;29:2330-9.
11. Maddox TM, Januzzi JL Jr, Allen LA, et al. Writing Committee. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol 2021;77:772-810.
12. Patoulias D, Papadopoulos C, Katsimardou A, Kalogirou MS, Doumas M. Meta-analysis Assessing the effect of sodium-glucose co-transporter-2 inhibitors on left ventricular mass in patients with type 2 diabetes mellitus. Am J Cardiol 2020;134:149-52.
13. Food and Drug Administration. FDA approves treatment for wider range of patients with heart failure. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-wider-range-patients-heart-failure [Last accessed on 19 Apr 2022].
14. European Commission. Union register of medicinal products for human use. Available from: https://ec.europa.eu/health/documents/community-register/html/h930.htm [Last accessed on 19 Apr 2022].
15. Berezin AE, Berezin AA. Shift of conventional paradigm of heart failure treatment: from angiotensin receptor neprilysin inhibitor to sodium-glucose co-transporter 2 inhibitors? Future Cardiol 2021;17:497-506.
16. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020;17:761-72.
17. Briasoulis A, Al Dhaybi O, Bakris GL. SGLT2 inhibitors and mechanisms of hypertension. Curr Cardiol Rep 2018;20:1.
18. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? Diabetes Obes Metab 2018;20:479-87.
19. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018;61:2108-17.
20. Nespoux J, Vallon V. Renal effects of SGLT2 inhibitors: an update. Curr Opin Nephrol Hypertens 2020;29:190-8.
21. Liu B, Wang Y, Zhang Y, Yan B. Mechanisms of protective effects of SGLT2 inhibitors in cardiovascular disease and renal dysfunction. Curr Top Med Chem 2019;19:1818-49.
22. Kolijn D, Pabel S, Tian Y, et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res 2021;117:495-507.
23. Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health 2019;16:2965.
24. Kelly MS, Lewis J, Huntsberry AM, Dea L, Portillo I. Efficacy and renal outcomes of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Postgrad Med 2019;131:31-42.
25. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. The Lancet Diabetes & Endocrinology 2019;7:845-54.
26. Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab 2019;21:1237-50.
27. Zou CY, Liu XK, Sang YQ, Wang B, Liang J. Effects of SGLT2 inhibitors on cardiovascular outcomes and mortality in type 2 diabetes: a meta-analysis. Medicine (Baltimore) 2019;98:e18245.
28. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819-29.
29. Petrie MC, Verma S, Docherty KF, et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA 2020;323:1353-68.
30. Packer M, Butler J, Filippatos GS, et al. EMPEROR-Reduced Trial Committees and Investigators. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail 2019;21:1270-8.
31. Szarek M, Bhatt DL, Steg PG, et al. SOLOIST-WHF committees and investigators. effect of sotagliflozin on total hospitalizations in patients with type 2 diabetes and worsening heart failure: a randomized trial. Ann Intern Med 2021;174:1065-72.
32. Anker SD, Butler J, Filippatos G, et al. EMPEROR-preserved trial investigators. empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451-61.
33. Yu YW, Zhao XM, Wang YH, et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiac structure and function in type 2 diabetes mellitus patients with or without chronic heart failure: a meta-analysis. Cardiovasc Diabetol 2021;20:25.
34. Dhingra NK, Mistry N, Puar P, et al. SGLT2 inhibitors and cardiac remodelling: a systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail 2021;8:4693-700.
35. Zhang N, Wang Y, Tse G, et al. Effect of sodium-glucose cotransporter-2 inhibitors on cardiac remodelling: a systematic review and meta-analysis. Eur J Prev Cardiol 2022;28:1961-73.
36. Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. EMPA-TROPISM (ATRU-4) Investigators. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021;77:243-55.
37. Lee MMY, Brooksbank KJM, Wetherall K, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation 2021;143:516-25.
38. Oldgren J, Laurila S, Åkerblom A, et al. Effects of 6 weeks of treatment with dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on myocardial function and metabolism in patients with type 2 diabetes: A randomized, placebo-controlled, exploratory study. Diabetes Obes Metab 2021;23:1505-17.
39. Singh JSS, Mordi IR, Vickneson K, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care 2020;43:1356-9.
40. McDonagh TA, Metra M, Adamo M, et al. ESC Scientific Document Group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726.[PMID:35373785 DOI:10.1714/3777.37630] Caution!
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Berezin AE, Berezin AA. Reversibility of adverse cardiac remodeling in type 2 diabetes mellitus patients: focus on sodium-glucose cotransporter-2 inhibitors . Vessel Plus 2022;6:56. http://dx.doi.org/10.20517/2574-1209.2021.141
AMA Style
Berezin AE, Berezin AA. Reversibility of adverse cardiac remodeling in type 2 diabetes mellitus patients: focus on sodium-glucose cotransporter-2 inhibitors . Vessel Plus. 2022; 6: 56. http://dx.doi.org/10.20517/2574-1209.2021.141
Chicago/Turabian Style
Berezin, Alexander E., Alexander A. Berezin. 2022. "Reversibility of adverse cardiac remodeling in type 2 diabetes mellitus patients: focus on sodium-glucose cotransporter-2 inhibitors " Vessel Plus. 6: 56. http://dx.doi.org/10.20517/2574-1209.2021.141
ACS Style
Berezin, AE.; Berezin AA. Reversibility of adverse cardiac remodeling in type 2 diabetes mellitus patients: focus on sodium-glucose cotransporter-2 inhibitors . Vessel Plus. 2022, 6, 56. http://dx.doi.org/10.20517/2574-1209.2021.141
About This Article
Copyright
Data & Comments
Data

 Cite This Article 7 clicks
Cite This Article 7 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.