Optimal perioperative care for thoracoabdominal and descending thoracic aortic aneurysm repair: a review
Abstract
In this review, the authors discuss the perioperative management of patients who undergo thoracoabdominal aortic aneurysm or descending thoracic aortic aneurysm repair. All major organ systems are potentially vulnerable to complications from these repairs; meticulous preoperative attention to optimizing relevant comorbidities, diligent performance of intraoperative anesthetic and surgical techniques, and careful postoperative management are necessary to maximize the likelihood of successful outcomes. Specific attention should be given to reducing the risk for spinal cord ischemia and for paraplegia. Of note, renal and respiratory systems are especially vulnerable to major complications and require a thoughtful multidisciplinary approach. Because preventing complications is the primary goal of perioperative management, deviations from the normal course must be recognized promptly and addressed aggressively to reduce the likelihood of major morbidity and death.
Keywords
INTRODUCTION
Achieving successful outcomes after thoracoabdominal aortic aneurysm (TAAA) or descending thoracic aortic aneurysm (DTA) repair requires meticulous attention throughout the entire perioperative period. This includes not only appropriate intraoperative technical considerations, but also preoperative optimization for patients with comorbidities, careful anesthetic management during the procedure, and diligent postoperative care[1]. Because preventing complications is the primary goal of perioperative management, deviations from the expected perioperative course must be recognized promptly and addressed aggressively to reduce the likelihood of deleterious consequences.
In this narrative review, we discuss typical characteristics of patients who require TAAA or DTA repair, the perioperative risks associated with surgery for these conditions, and the management of associated organ systems so as to reduce morbidity. In addition, we consider unique aspects of managing endovascular repair of TAAAs and DTAs. Our literature review included the following search terms: thoracoabdominal aortic surgery, descending thoracic aortic aneurysm, and thoracic endovascular aortic repair. Our discussion is based on our collective experience of more than 4000 open repairs over the last 3 decades.
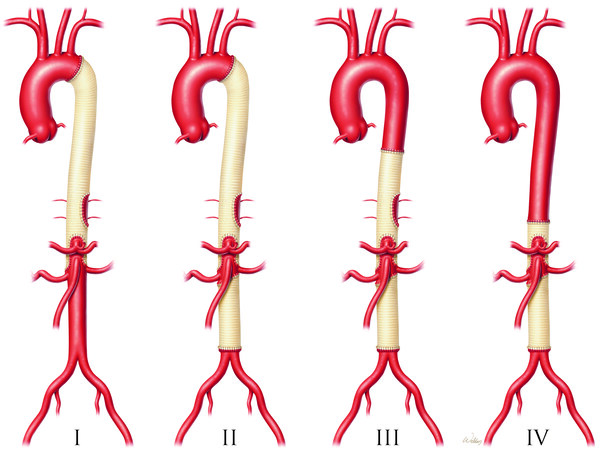
It is common for patients to have coexisting medical conditions that should be evaluated before TAAA repair. Special attention should be given to optimizing those comorbidities preoperatively, followed by organ system-specific evaluation and discussion of management considerations. Broadly speaking, two general categories of patients require TAAA repair: (1) younger patients who are likely to have Marfan syndrome or other connective tissue disorders and who frequently have had previous aortic surgery, present with a higher proportion of Crawford extent I or II aneurysms, and have fewer medical comorbidities; and (2) older patients presenting with degenerative atherosclerotic aortic aneurysms and typically having a higher proportion of Crawford extent IV aneurysms, less-frequent previous aortic surgery, and more medical comorbidities. See Figure 1[2].
Figure 1. E. Stanley Crawford classification of thoracoabdominal aortic aneurysm repair by extent of involvement. Extent I repairs involve most or all of the descending thoracic aorta and the upper abdominal aorta. Extent II repairs involve the same segments as extent I repairs but also extend into the infrarenal abdominal aorta. Extent III repairs involve a combination of the distal half, or less, of the descending thoracic aorta (beginning below the sixth rib) and varying portions of the abdominal aorta. Extent IV repairs involve the abdominal aorta below the diaphragm. Image created by Scott A. Weldon, MA, CMI, FAMI and printed with permission from Baylor College of Medicine.
PREOPERATIVE EVALUATION AND CONSIDERATIONS
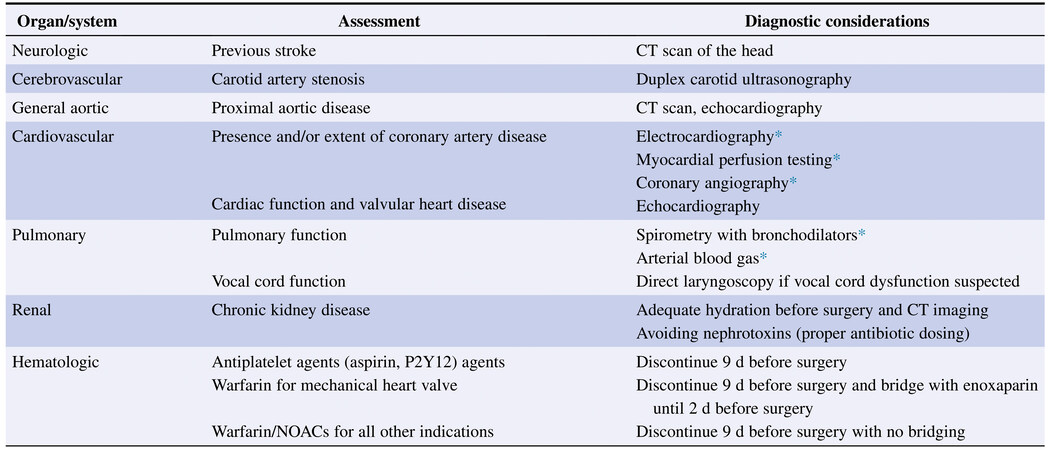
Standardized procedures for the preoperative evaluation of patients scheduled for surgical TAAA or DTA repair are available from professional organizations such as the American Society of Anesthesiologists and the European Society of Anesthesiology and Intensive Care. Preoperative assessment should emphasize evaluation of airway, cardiac, pulmonary, and neurological systems, preoperative laboratory tests, and imaging[3]. A listing by organ system and diagnostic preoperative considerations is provided in Figure 2[1]. Although age itself is not a contraindication to open TAAA or DTA repair, the operative mortality risk in octogenarians is 10-fold higher than in younger patients and warrants careful patient selection[4]. Perhaps unsurprisingly, preoperative functional status has been shown to be the strongest independent predictor of perioperative death, with completely dependent functional status yielding a threefold-higher mortality risk[5]. Thus, baseline functional status and mobility should be observed in all patients.
Figure 2. Preoperative considerations by organ system for assessment and diagnostic evaluation before TAAA or DTA repair. CT: Computed tomography; DTA: descending thoracic aneurysm; NOAC: non-vitamin K oral anticoagulant (dabigatran, rivaroxaban, apixaban, edoxaban); TAAA: thoracoabdominal aortic aneurysm. *As appropriate. Reprinted from the ref.[1], with permission from Elsevier.
Neurological (cerebral)
Neurological deficits can develop in TAAA and DTA patients not only as sequelae of underlying cerebrovascular disease, but also as complications of surgical repair. Careful preoperative screening can be helpful in preventing such complications. For example, in our institutional experience, 10% of patients presenting for TAAA or DTA repair have evidence of previous stroke. Neurological consultation should be requested when indicated, and preoperative imaging should be obtained in selected patients as a baseline reference.
Preoperative neurological assessment should include a physical exam to detect baseline neurological deficits, duplex ultrasonography of the carotid arteries in patients older than age 60, and computed tomographic angiography with 3-dimensional reconstruction to evaluate the size and contour of the aorta, detect the presence of thrombus or atheroma, and identify any anatomical variants - for example, atypical location of major spinal cord branches such as the artery of Adamkiewicz (which is present in 85% of the population, on the left side more than 75% of the time and between T8 and L1 in approximately 90% of people[6]) or collateral blood flow that could affect the surgical approach. Treatment of symptomatic or severe asymptomatic carotid occlusive disease should be considered before proceeding with TAAA or DTA repair, depending on the severity of the cerebrovascular disease and the urgency of the thoracic aortic intervention.
Patients who undergo repair under hypothermic circulatory arrest have a greatly increased incidence of stroke, up to 9%[7]. The typical indications for circulatory arrest are rupture and aneurysm anatomy unsuitable for clamping from the thoracoabdominal incision. Otherwise, the use of this technique is rare in our clinical practice. Significant dementia or other neurological conditions may contraindicate surgical repair and should be discussed with the patient’s family.
Neurological (spinal cord)
Spinal cord injury resulting from inadequate spinal cord perfusion during TAAA or DTA repair occurs in up to 20% of patients, with higher rates associated with Crawford extent II and III aneurysms; 2% to 3% of these patients will develop permanent paresis or paraplegia[7-9]. Sarcopenia and associated frailty have been associated with increased risk for paraplegia[10]. Assessment for gait speed and grip strength are included in a frailty assessment to better delineate the risks of surgery. Patients are counseled in detail and in advance about the risk for spinal cord injury, given its life-altering impact.
Pulmonary
Concurrent pulmonary disease is present in 35% to 45% of TAAA patients[7]. Ongoing tobacco use is widely prevalent among these patients (79%); consequently, chronic obstructive pulmonary disease (COPD) has been shown to be significantly more frequent (1.8-fold) in patients with aortic aneurysms than in those without[7,11,12]. At least two studies have shown that COPD is associated with aortic aneurysm rupture[12,13]. Moreover, the pain caused by the large incision across the thoracic and abdominal cavities can increase the risk for atelectasis and pulmonary complications.
Patients should undergo preoperative pulmonary workup to establish their ability to tolerate open surgery and prolonged intraoperative single-lung ventilation, which is required for TAAA repair. Preoperative pulmonary function should be evaluated with spirometry, diffusing capacity for carbon monoxide, and, in higher-risk patients, arterial blood gas assessment of oxygenation and ventilation. Smoking history should be obtained, and counseling for immediate smoking cessation should be provided. Patients who previously underwent aortic arch or descending thoracic aortic surgery may already experience hoarseness; direct laryngoscopy by an otorhinolaryngologist should be obtained for baseline assessment of vocal cord mobility. Higher-risk patients are candidates for preoperative pulmonary optimization with inhaled bronchodilators and/or steroids, risk stratification for postoperative prolonged ventilation, or consideration of pharmacological adjuvants (such as inhaled pulmonary vasodilators). In cases of severe pulmonary dysfunction, reconsideration of the appropriateness of surgery and the feasibility of an endovascular alternative is warranted.
Pleural effusion, although more frequently present in cases of acute aortic dissection, is a known presentation in patients with a DTA or TAAA. Large effusions usually indicate aneurysm dissection or rupture with hemorrhage into the pleural space, whereas smaller effusions are predominantly exudative and inflammatory[14]. Large TAAAs can compress adjacent structures, including pulmonary vessels, leading to unilateral pulmonary edema and dyspnea. Rarely, aortopulmonary fistulas can be caused by aneurysm erosion into lung parenchyma, potentially causing massive hemoptysis, respiratory failure, and hemorrhagic shock[14,15].
Cardiovascular
Coexisting cardiac disease, especially coronary artery disease, is prevalent in approximately 30% of extent I and II patients and approximately 50% of extent III and IV patients[1]. In our 2016 case series, 15% of patients undergoing open TAAA repair had previously undergone coronary artery bypass grafting[7]. Of particular importance is preoperative assessment of any left internal mammary graft, as clamping during repair of extent I or II aneurysms can injure the graft or occlude its takeoff from the left subclavian artery. Moreover, aortic cross-clamping without left-heart bypass greatly increases afterload on the heart, which in turn amplifies myocardial oxygen demand. When the coronary blood supply is not capable of sustaining this increased demand, myocardial ischemia results. Postoperatively, coronary ischemia can be caused by blood loss, graft or stent occlusion, or embolic events. The extent of coronary artery disease and urgency of the TAAA or DTA will influence the choice of revascularization strategy and the timing of the repair.
Detecting coexisting cardiac disease requires a comprehensive cardiac evaluation, starting with baseline electrocardiography, echocardiographic evaluation of ventricular and valvular function, and, if warranted, a myocardial perfusion scan and cardiac catheterization[16]. Patients should be risk-stratified for perioperative cardiac complications by using predictive tools such as the Revised Cardiac Risk Index[17], which considers the type of surgery and history of ischemic heart disease, heart failure, cerebrovascular disease, diabetes, and preoperative renal failure to estimate 30-day risk for death, myocardial infarction, and cardiac arrest. In our practice, we follow current guidelines to help guide further workup and diagnostic testing[18].
For patients presenting with acute dissection or rupture, anti-impulse therapy is promptly initiated with intravenous labetalol or esmolol as first-line agents; other vasodilators (nicardipine) are administered as needed.
Renal
Acute kidney injury (AKI) is a common complication of thoracic aortic surgery, with higher rates in extent II repairs[7]. Preoperative renal dysfunction, advanced age, long intraoperative renal ischemia times due primarily to aortic clamping and renal artery disruption, and transfusion of packed red cells are known risk factors for postoperative AKI[7,19,20]. Reported postoperative dialysis rates range from 2% to 12%[21]. Chronic kidney disease (CKD) considerably increases the risk for renal replacement therapy and early death[22]. Permanent renal failure with dialysis is associated with a significant increase in mortality, with one large case series reporting an in-hospital mortality rate of 57%[7]. Thus, in patients with CKD, preoperative nephrology consultation and discussion of the potential need for renal replacement therapy is worthwhile.
Close preoperative assessment of renal function is warranted. If a patient is on dialysis that uses an arteriovenous fistula or graft, we place a temporary dialysis catheter at the time of surgery to provide for continuous renal replacement therapy in the postoperative period. This is done to minimize the hemodynamic effects of premature initiation of hemodialysis during this early postoperative period, which may induce hypotension and risk spinal cord ischemia. Similarly, a temporary dialysis catheter is routinely placed in the operating room for patients at increased risk for needing dialysis (primarily those with advanced CKD). A dialysis catheter placed femorally can be relocated to an alternative site as needed to reduce the risk for postoperative infection.
Hematological
Because patients undergoing TAAA or DTA repair are at significant risk for hemorrhage, preoperative hematological analysis is essential. Serum hemoglobin, blood type and screen, platelet count, and measures of coagulation status - including partial thromboplastin time, prothrombin time, and fibrinogen level - should be routinely checked preoperatively, including on the day of surgery. Supplemental tests, such as platelet aggregation to evaluation function, may be indicated in select patients. Any abnormalities should be corrected before the operation.
The preoperative management of antiplatelet or antithrombotic agents should proceed in accordance with guidelines from the American College of Cardiology/American Heart Association and the American Society of Regional Anesthesia, especially in patients who will require a cerebrospinal fluid (CSF) drainage catheter to prevent procedural complications, such as spinal epidural hematomas[23]. If a patient with a mechanical prosthetic valve is taking a vitamin K antagonist, the international normalized ratio (INR) is allowed to drift down. When indicated, more-rapid correction can be achieved with vitamin K or prothrombin complex concentrate (KCentra; CSL Behring, King of Prussia, PA).
For elective cases, warfarin is held for a minimum of 9 days preoperatively; enoxaparin is used as a bridge in selected patients with mechanical valves and is stopped 48 h before surgery. In inpatient cases, an unfractionated heparin drip will allow for rapid reversal as needed.
INTRAOPERATIVE CONSIDERATIONS
As a high-risk, technically challenging endeavor, TAAA or DTA repair - especially for Crawford extent II aneurysms - can cause potentially serious complications, such as intraoperative death, paraplegia, and renal failure. Protective surgical techniques should be employed to mitigate the extent of injury[24]. Cardiac anesthesiologists require an in-depth understanding of the steps involved in order to anticipate and mitigate physiological disturbances during periods of hemodynamic instability[7,24].
Before surgery, the blood bank should be notified for typing and cross-matching of 6 units of packed red blood cells, 6 units of fresh frozen plasma, at least 1 or 2 units of platelets (more may be warranted, depending on initial platelet count and previous antiplatelet medications), and cryoprecipitate. These products should be available before surgical incision, with the understanding that delays may arise due to preexisting antibodies[24].
Cerebrospinal fluid drainage
Spinal cord injury may be caused by periods of hypoperfusion and subsequent reperfusion injury. The risk for this devastating complication is high during TAAA and DTA repairs, and preventing it remains a focus of ongoing research. Placing a CSF drain is an American College of Cardiology, American Heart Association, and American Association for Thoracic Surgery class I recommendation for any open or endovascular repair in patients at high risk for spinal cord injury, so long as there is no contraindication to placement[25]. A CSF drain allows for careful maintenance of adequate spinal cord perfusion pressure (SCPP), which is the difference between mean arterial pressure (MAP) and intraspinal pressure (ISP). In our randomized trial in patients with extent I or II aneurysms, CSF drainage was associated with an 80% reduction in the incidence of postoperative deficits[26].
Practices regarding the timing and location for elective placement of a CSF drain vary across institutions. Some institutions prefer placement in the preoperative holding area, with the patient sitting and leaning forward, similar to the approach used for labor and thoracic epidural placement[27]. Other institutions prefer intraoperative placement after the patient has been induced and intubated and central lines have been placed, with the patient placed in right lateral decubitus position; this is our preferred approach[24,28]. Drain placement can be deferred until after the operation for unstable patients and patients for whom drain placement would be difficult. In selected patients, such as those with spinal instrumentation, the CSF drain can be placed under radiographic guidance. Barring complications, CSF drains are generally removed on the second or third postoperative day. In patients who lack a reliable neurological exam, such as comatose patients, the drain may be left in longer.
A CSF drain is placed under sterile conditions. The anatomical landmarks are palpated, and a 14G Tuohy needle is inserted and advanced into either the L2-L3, L3-L4, or L4-L5 space until clear CSF is acquired. The lumbar drainage catheter is then advanced into the subarachnoid space, secured with sterile dressings, connected to the CSF drainage system, and transduced to obtain a baseline CSF pressure. Intraoperative adjuncts such as naloxone have shown promise in animal models but have not been shown to have reliable clinical benefit in human trials[29]. Close monitoring of the MAP from the arterial line and the ISP from the CSF drain is used to maintain the SCPP at > 70-75 mmHg. The MAP is routinely maintained at
Anesthesia management and monitoring
Patients undergoing TAAA or DTA repair are transported to the operating room, transferred to the table, and placed supine onto a bean bag device that will later serve to maintain the surgical position. The standard American Society of Anesthesiologists monitors[31] are placed and supplemented with invasive monitoring. Cerebral oximetry monitors are placed to obtain a baseline reading before preoxygenation. Premedication is then administered to ease arterial cannulation. A right radial or brachial arterial catheter is placed before anesthesia is administered; this is preferred over a left upper-extremity arterial line, which can be compromised during aortic cross-clamping if the left subclavian artery also is clamped. At that critical juncture, accurate measurement of arterial blood pressure is paramount.
After adequate preoxygenation, general anesthesia is induced using a patient-specific combination of lidocaine, etomidate, propofol, an opiate such as fentanyl, and a muscle relaxant, with the goal of avoiding hemodynamic lability during intubation. Use of the short-acting beta-blocker esmolol or the vasodilator nitroglycerin may be required to avoid significant tachycardia and hypertension, which increase aortic wall stress and tension and the likelihood of subsequent aortic dissection or aneurysm rupture[24,28].
The surgical team should notify the anesthesia team if the preoperative computed tomography (CT) scan suggests bronchial or tracheal compression by the aneurysm, which may necessitate modifying the airway strategy. Intubation is typically performed using a double-lumen endotracheal tube or, when intraoperative single-lung ventilation is required, a single-lumen endotracheal tube with a left-sided bronchial blocker. Correct positioning is confirmed by using fiberoptic bronchoscopy. Anesthesia maintenance involves a balanced technique with volatile anesthesia, opiate, and dexmedetomidine infusion. Patients whose diffusing capacity of carbon monoxide is severely reduced may require total intravenous anesthesia; however, this could cause the loss of beneficial volatile anesthesia-induced cardioprotection.
Generally, central venous access is obtained by cannulating the internal jugular vein with a large-bore double-lumen catheter, which provides a central port for pulmonary artery catheter placement. We recommend routine use of pulmonary artery catheters, along with monitoring of the cardiac index and mixed venous oxygen saturation. Pulmonary artery catheter placement not only assists in the intraoperative assessment of cardiac function, volume status, and pulmonary pressures (especially during single-lung ventilation), but also can be invaluable in managing a hemodynamically unstable patient postoperatively. Our center recommends concomitant use of a large-bore triple-lumen cannula and a second large-bore single-lumen sheath through which the pulmonary artery catheter can be placed, given that rapid transfusion may be necessary[24]. To facilitate rapid infusion at rates > 500 mL/min, we use the Belmont Rapid Infuser FMS2000 (Belmont Medical Technologies, Billerica, MA) and cell-salvage blood conservation[32]. Central lines that are not being utilized are removed promptly in the postoperative period to reduce the risk for infection.
Intraoperative transesophageal echocardiography should be considered for assessing cardiac function and volume status during periods of hemodynamic instability. It can also be used to assist in placing left heart bypass cannulas. Transesophageal echocardiography should be used in patients with preexisting coronary disease or heart failure (left ventricular ejection fraction < 40%), as echocardiography is more sensitive than electrocardiography in detecting myocardial ischemia[27].
POSTOPERATIVE MANAGEMENT AND CONSIDERATIONS
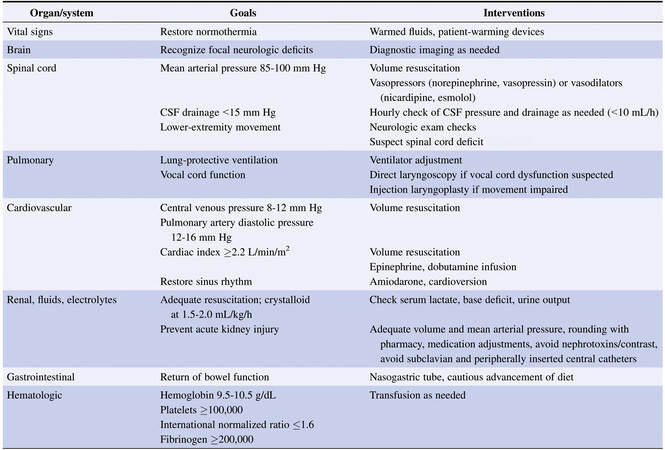
The postoperative course of patients who have undergone open TAAA or DTA repair is often characterized by coagulopathy, reperfusion injury, and risk for bleeding and/or ischemia[2]. In a study of 3309 patients, we found that operative mortality for TAAA repair ranged from 5% to 10%, depending on preexisting comorbidities and the anatomical extent of the aneurysm[7]. Other large series consisting of more than 500 combined TAAA and DTA repairs reported operative mortality rates of 6% to 16%[33-35]. As expected, the highest risk is with extent II aneurysm repair. A detailed procedure for patient handoff from the surgical and anesthesia teams to the intensive care unit (ICU) team is essential for proper communication and optimal continuity of care[36]. The fundamental areas of focus in the immediate postoperative period are maintaining adequate MAP, volume resuscitation, rewarming, and expectant management of potential complications. An example of a system-by-system approach to postoperative care is shown in Figure 3[2].
Figure 3. Postoperative considerations by organ system for therapeutic goals and interventions after TAAA or DTA repair.
Neurological (cerebral)
As with any operation involving manipulation of the aorta, there is the possibility of stroke. The overall risk for stroke in the acute perioperative period for patients undergoing TAAA repair is about 2% to 3%[7]; the risk is highest with extent II aneurysms and lowest with extent III aneurysms. Most strokes are ischemic or embolic, with only a small minority being hemorrhagic. Embolic stroke is caused either by dislodgement of atheromatous debris or entrainment of air during surgery. Fluctuations in blood pressure can induce hypotensive stroke, particularly during reperfusion or if significant bleeding develops. Approximately one-quarter of patients noted to have stroke symptoms will recover without deficits[7].
After the patient awakens from anesthesia, any focal neurologic deficits should be quickly evaluated with noncontrast head CT to exclude a hemorrhagic cause. Prompt neurosurgical consultation should be obtained if hemorrhage has been noted.
Neurological (spinal cord)
Spinal cord deficits are the most feared major morbidity after TAAA or DTA repair, particularly after operations for extent II or III aneurysms. The risk for spinal cord deficit has decreased significantly in recent years: In 1991, Crawford’s group reported that the incidence of spinal cord deficit in 1509 TAAAs was 16% overall and as high as 31% in patients needing extent II repairs[37]; by 2016, the overall rate had decreased to 9.6%, and only 2.9% of patients developed persistent paraplegia[7].
It is critical to ensure adequate volume resuscitation (as guided by central venous or pulmonary artery diastolic pressure) in these patients, particularly those with high blood loss. Evaporative or third-space losses during surgery are often underestimated, and this is particularly true for TAAA repair, which involves a large incision with viscera and lung exposed for several hours. The postoperative MAP goal varies among institutions; our usual goal for these patients is 85-100 mmHg, once any significant bleeding has been controlled[38].
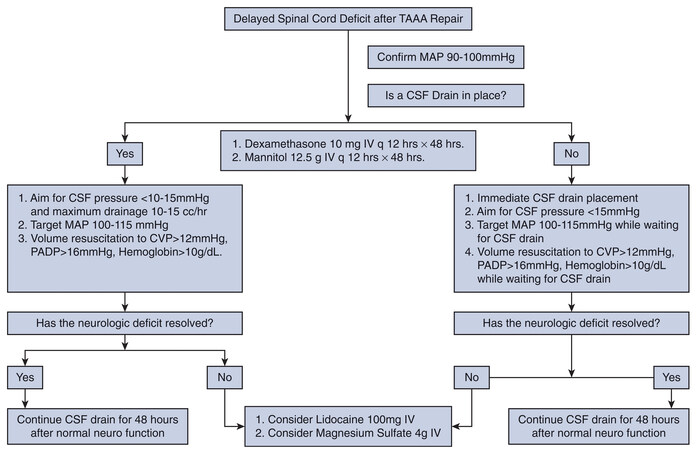
Any postoperative neurological deficit portending possible spinal cord injury warrants a further increase in SCPP by increasing MAP, which necessitates frequent CSF drainage (in 10 mL/h aliquots, not to exceed
Figure 4. Management algorithm for delayed paraplegia after thoracoabdominal aortic aneurysm repair. CSF: Cerebrospinal fluid; CVP: central venous pressure; IV: intravenous; MAP: mean arterial pressure; PADP: pulmonary artery diastolic pressure; TAAA: thoracoabdominal aortic aneurysm. Reprinted from the ref.[2], with permission from Elsevier.
Approximately 10% of patients have severe headache related to the CSF drain[40]. Caffeine ingestion or an epidural blood patch, as is used for postdural puncture headache, is effective. Up to 5% of patients have severe complications, including epidural hematoma, meningitis, or neurological deficit, related to the CSF drainage catheter or its placement[41,42]. Inattention to detail in a patient with a CSF drain can cause catastrophic central nervous system injury or herniation. All staff taking care of the patient should be well-trained in the use of CSF drains and the complications that can develop.
Pulmonary
In our practice, patients usually arrive in the ICU with a dual-lumen endotracheal tube in place in case reoperation for bleeding is necessary. Mechanical ventilation with lung-protective tidal volume settings and a higher positive end-expiratory pressure is employed to promote recruitment of the left lung[38]. Liberation from mechanical ventilation should be done only after the patient has been warmed, has no significant bleeding, and has near-normal acid-base status and adequate resuscitation. We typically aim for extubation the morning after surgery, having maintained head-of-bed elevation overnight to reduce airway swelling. If extubation within the first 24 h appears unlikely, the dual-lumen endotracheal tube is exchanged for a single-lumen tube, which facilitates pulmonary hygiene and allows for therapeutic bronchoscopy if needed.
After extubation, close attention should be paid to voice quality. Injury to the recurrent laryngeal nerve occurs in up to a quarter of patients undergoing extent I or II aneurysm repair[41,42]. The recurrent nerve loops around the aortic arch close to the proximal clamping site and can be injured by dissection, the clamp, or traction on nearby structures. If the patient has altered voice or cough after extubation, an otolaryngology consultation should be requested, and direct laryngoscopy should be performed. For patients with impaired vocal-cord movement, transcervical injection laryngoplasty can be effective[43]. This can be done in the ICU without general anesthesia. Bedside swallow evaluation should be done if impaired vocal-cord movement is diagnosed to determine whether diet advancement is appropriate or if the patient is at higher risk for aspiration.
Tracheostomy has been reported in up to 12% of TAAA repairs - unsurprising, given the extensive pulmonary comorbidities in this patient population. Tracheostomy timing and technique (open versus percutaneous dilational) should follow local practice. Early tracheostomy (< 14 days after surgery) is recommended to facilitate mobility, reduce the risk for pneumonia, and promote ventilator weaning[44]. Venovenous extracorporeal membrane oxygenation has been used successfully in cases of severe hypoxemia[45].
Cardiovascular
In the immediate postoperative setting, we routinely use a pulmonary artery catheter to maintain a cardiac index of > 2.2 L/min/m2. We administer epinephrine or dobutamine for inotropic support. Cardiac output and various perfusion indices (serum lactate, urine output, mixed venous oxygen saturation, base deficit, and overall hemodynamics) are monitored to gauge resuscitation.
Up to one-quarter of patients undergoing TAAA repair develop atrial fibrillation. The incidence is highest in patients of advanced age and those requiring visceral perfusion intraoperatively[46]. Atrial fibrillation can lead to hypotension and risks spinal cord ischemia. We recommend an aggressive rhythm-control strategy with intravenous amiodarone and early electrical cardioversion if needed.
Severe hypertension (systolic blood pressure > 160-170 mmHg) should be avoided. However, the risk for delayed paraplegia can persist for a month after surgery[47]. For that reason, strict hypertension management should not begin until after 1 month postoperatively, in consultation with the patient’s primary care physician and cardiologist.
Peripheral vascular
Postoperatively, the peripheral pulses are monitored closely. Any change in the pulse exam from preoperative and immediate postoperative status requires prompt evaluation with Doppler ultrasonography and timely intervention if needed. As with any operation involving prolonged lower-extremity ischemia and reperfusion, compartment syndrome and rhabdomyolysis could develop; if so, prompt intervention with four-compartment fasciotomies is necessary for limb salvage. Lower-extremity ischemia can also be caused by a nonpatent aortoiliac anastomosis, in which case endovascular intervention or graft revision may be necessary.
Renal
Both TAAA repair and DTA repair are fraught with various insults to the kidneys. In a narrative review of nearly 9000 TAAA repairs, the incidence of AKI ranged from 5% to 29%, and the percentage of patients requiring dialysis was between 4% and 17%[21]. Moderate AKI portends increased mortality[21], and even mild AKI is associated with a greatly increased need for tracheostomy. In patients with renal failure requiring dialysis who also require tracheostomy, mortality is as high as 70%[21]. Many of the strategies for preventing AKI (e.g., volume resuscitation, adequate MAP) overlap with methods used to prevent spinal cord ischemia.
Multidisciplinary rounding with a pharmacist is invaluable for avoiding iatrogenic medication-related exacerbation of AKI. Early nephrology consultation should be considered for any patient who develops AKI. Close adherence to the guidelines suggested by the Kidney Disease Improving Global Outcomes (KDIGO)[48] initiative is critical. The use of contrast imaging should be carefully weighed against the risk for contrast nephropathy that could exacerbate preoperative CKD or postoperative AKI, and adequate hydration should be maintained. Peripheral insertion of catheters is avoided whenever possible to preserve future dialysis access options in the upper extremities.
Gastrointestinal
Early gastrointestinal complications after TAAA or DTA repair are not common (6%) but are associated with a considerable increase in operative mortality (34%) and major morbidity[49]. As with abdominal surgery in general, the most common gastrointestinal complication after TAAA or DTA repair is ileus. With ileus, opioids and anticholinergics should be avoided as much as possible, and the patient’s electrolytes should be kept in a normal range. Stress-ulcer prophylaxis should be routine after TAAA or DTA repair.
Unexplained acidosis, excessive fluid requirements, or abdominal pain and distension may indicate mesenteric ischemia. Although CT angiography may be necessary, mesenteric ischemia often occurs in the setting of AKI, and the possibility of contrast nephropathy must be considered. If clinical suspicion is strong, prompt laparotomy should be considered. Along those lines, abdominal compartment syndrome also is a consideration, especially in patients with a ruptured aneurysm at the time of presentation, and appropriate bladder pressure monitoring in the appropriate clinical context is essential for timely management[50]. In addition, significant elevation in hepatic transaminases should prompt Doppler ultrasonography to exclude mesenteric vascular thrombosis.
Enteral nutrition should be initiated as soon as possible and when safe, usually on or after the third postoperative day after removal of the nasogastric tube. For patients who remain intubated or who are deemed unsafe for oral intake, enteral feeds via nasoduodenal tube should be given.
Patients who undergo splenectomy during their procedure should be vaccinated against Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis infection[51]. If not administered preoperatively, these vaccinations should be given within 2 weeks postoperatively. Patients who have undergone splenectomy are also at risk for injury to the tail of the pancreas. Suspicious abdominal drain output or feeding intolerance warrants evaluation of serum pancreatic enzymes. Postoperative pancreatitis should be treated in a standard manner, with bowel rest.
Hematological
Management of postoperative bleeding should focus on correcting coagulopathy, systemic warming, and prompt return to the operating room when indicated. Our postoperative targets include a platelet count
UNIQUE CONSIDERATIONS FOR Endovascular Repair of the desceNDing thoracic aorta
The introduction of endovascular stent-grafts in 2005 has led to widespread adoption of thoracic endovascular aortic repair (TEVAR) for DTAs. As a less-invasive procedure than open repair, TEVAR significantly reduces the physiological insult to the patient, reduces the early mortality rate, and is associated with a lower risk for many of the complications associated with open repair, such as paraplegia, renal and respiratory failure, the need for transfusions, and longer hospital stays, among others[53]. For example, most patients undergoing TEVAR are extubated on the operating room table. Nonetheless, TEVAR raises several unique considerations in perioperative care[54].
Postimplantation syndrome
Postimplantation syndrome is characterized by a systemic inflammatory response, with elevation of inflammatory cytokines and other inflammation markers. Sympathetic pleural effusions may also be present. The reported incidence of postimplantation syndrome varies widely (from 13% to 60%)[55]. The syndrome is thought to be caused by local inflammatory cascade in the endothelium of the excluded aorta. Postimplantation syndrome does not require antimicrobial therapy (although differentiation from infection may be difficult). Treatment consists of aspirin and vigilant monitoring for other causes of systemic inflammatory response.
Spinal cord management
In TEVAR, the spinal cord is managed much as it is for open repair. With short-segment coverage from the endograft, the risk for spinal cord deficit is minimized. However, as the length of the TEVAR graft increases, the risk for spinal cord ischemia increases[56]. Cerebrospinal fluid drains are used less frequently in TEVAR than in open surgery, and the data supporting the effectiveness of CSF drains in TEVAR are less robust. We do selectively use CSF drains in patients undergoing long-segment coverage of the descending thoracic aorta and those with previous abdominal aortic aneurysm repair. In patients undergoing TEVAR without a CSF drain, any signs of spinal cord ischemia should prompt immediate drain placement and management, as described for open repair.
Left subclavian artery management
Whether to revascularize the left subclavian artery or not is controversial, as there is debate as to whether the risk for stroke is increased or decreased by subclavian revascularization[57]. However, revascularization is essential in certain patients, such as those with patent left internal mammary artery conduits or dominant left vertebral arteries. In our practice, we revascularize the covered left subclavian artery in many, but not all, patients. When in doubt, we perform the TEVAR and then obtain noninvasive measurements of the left hand, including pulse oxygen waveform, photoplethysmography, and brachial-brachial index, before deciding whether to proceed with revascularization. As in patients with steal syndrome after arteriovenous fistula creation, mild symptoms can be managed using a structured exercise program.
CONCLUSION
The myriad conditions that complicate open TAAA and DTA repairs require specialized surgical expertise and focused attention to perioperative care. Achieving successful outcomes after TAAA or DTA repair requires knowledge about how to prevent complications and the ability to promptly recognize complications to mitigate the extent of the injury. A multidisciplinary team approach and clear communication are necessary to achieve these results.
DECLARATIONS
AcknowledgmentsThe authors thank Jeanie F. Woodruff, BS, ELS, of the Scientific Publications Department of The Texas Heart Institute, for her editorial contributions.
Authors’ contributionsMade substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; gave final approval of the version to be published; agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestPreventza O. serves as a consultant for Terumo Aortic and W.L. Gore & Associates. Coselli J.S. participates in clinical studies with and/or consults for Terumo Aortic, Medtronic, W. L. Gore & Associates, CytoSorbents, Edwards Lifesciences, and Abbott Laboratories and receives royalties and grant support from Terumo Aortic. Moon M.R. serves on the advisory board for Medtronic. Chatterjee S. has served on advisory boards for Edwards Lifesciences, La Jolla Pharmaceutical Company, Eagle Pharmaceuticals, and Baxter Pharmaceuticals. The remaining authors have no potential conflicts of interest related to this work.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Chatterjee S, Casar JG, LeMaire SA, Preventza O, Coselli JS. Perioperative care after thoracoabdominal aortic aneurysm repair: the Baylor College of Medicine experience. Part 1: preoperative considerations. J Thorac Cardiovasc Surg 2021;161:693-8.
2. Chatterjee S, Casar JG, LeMaire SA, Preventza O, Coselli JS. Perioperative care after thoracoabdominal aortic aneurysm repair: the Baylor College of Medicine experience. Part 2: postoperative management. J Thorac Cardiovasc Surg 2021;161:699-705.
3. Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012;116:522-38.
4. Aftab M, Songdechakraiwut T, Green SY, et al. Contemporary outcomes of open thoracoabdominal aortic aneurysm repair in octogenarians. J Thorac Cardiovasc Surg 2015;149:S134-41.
5. Obeid T, Hicks CW, Yin K, et al. Contemporary outcomes of open thoracoabdominal aneurysm repair: functional status is the strongest predictor of perioperative mortality. J Surg Res 2016;206:9-15.
6. Taterra D, Skinningsrud B, Pękala PA, et al. Artery of Adamkiewicz: a meta-analysis of anatomical characteristics. Neuroradiology 2019;61:869-80.
7. Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2016;151:1323-37.
8. Parotto M, Ouzounian M, Djaiani G. Spinal cord protection in elective thoracoabdominal aortic procedures. J Cardiothorac Vasc Anesth 2019;33:200-8.
9. Gaudino M, Khan FM, Rahouma M, et al. Spinal cord injury after open and endovascular repair of descending thoracic and thoracoabdominal aortic aneurysms: a meta-analysis. J Thorac Cardiovasc Surg 2022;163:552-64.
10. Chatterjee S, Shi A, Yoon L, et al. Effect of sarcopenia on survival and spinal cord deficit outcomes after thoracoabdominal aortic aneurysm repair in patients 60 years of age and older. J Thorac Cardiovasc Surg 2021; doi: 10.1016/j.jtcvs.2021.05.037.
11. Takagi H, Umemoto T, ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. A meta-analysis of the association of chronic obstructive pulmonary disease with abdominal aortic aneurysm presence. Ann Vasc Surg 2016;34:84-94.
12. Cronenwett JL, Murphy TF, Zelenock GB, et al. Actuarial analysis of variables associated with rupture of small abdominal aortic aneurysms. Surgery 1985;98:472-83.
13. Juvonen T, Ergin MA, Galla JD, et al. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg 1997;63:1533-45.
14. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369.
15. Inam H, Zahid I, Khan SD, Haq SU, Fatimi S. Hemoptysis secondary to rupture of infected aortic aneurysm - a case report. J Cardiothorac Surg 2019;14:144.
16. Girardi LN, Rabotnikov Y, Avgerinos DV. Preoperative percutaneous coronary intervention in patients undergoing open thoracoabdominal and descending thoracic aneurysm repair. J Thorac Cardiovasc Surg 2014;147:163-8.
17. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043-9.
18. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2215-45.
19. Godet G, Fléron MH, Vicaut E, et al. Risk factors for acute postoperative renal failure in thoracic or thoracoabdominal aortic surgery: a prospective study. Anesth Analg 1997;85:1227-32.
20. Acher C, Wynn M. Outcomes in open repair of the thoracic and thoracoabdominal aorta. J Vasc Surg 2010;52:3S-9S.
21. Chatterjee S, LeMaire SA, Amarasekara HS, et al. Early-stage acute kidney injury adversely affects thoracoabdominal aortic aneurysm repair outcomes. Ann Thorac Surg 2019;107:1720-6.
22. Coselli JS, Amarasekara HS, Zhang Q, et al. The impact of preoperative chronic kidney disease on outcomes after Crawford extent II thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2018;156:2053-64.e1.
23. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (fourth edition). Reg Anesth Pain Med 2018;43:263-309.
24. Coselli JS, de la Cruz KI, Preventza O, LeMaire SA, Weldon SA. Extent II thoracoabdominal aortic aneurysm repair: How I do it. Semin Thorac Cardiovasc Surg 2016;28:221-37.
25. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129.
26. Coselli JS, LeMaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9.
27. Chaudhary O, Sharkey A, Schermerhorn M, et al. Protocolized based management of cerebrospinal fluid drains in thoracic endovascular aortic aneurysm repair procedures. Ann Vasc Surg 2021;72:409-18.
28. Anton JM, Herald KJ. Anesthetic management of open thoracoabdominal aortic aneurysm repair. Int Anesthesiol Clin 2016;54:76-101.
29. Vandiver MS, Vacas S. Interventions to improve perioperative neurologic outcomes. Curr Opin Anaesthesiol 2020;33:661-7.
30. Chatterjee S, Preventza O, Mousavi MC, Orozco-Sevilla V, LeMaire SA, Coselli JS. Successful use of angiotensin II for vasoplegia after thoracoabdominal aortic aneurysm repair. JTCVS Tech 2020;4:72-5.
31. American Society of Anesthesiologists. Standards for basic anesthetic monitoring. Available from: https://www.asahq.org/standards-and-guidelines/standards-for-basic-anesthetic-monitoring. [Last accessed on 24 Jun 2022].
32. Klein AA, Bailey CR, Charlton AJ, et al. Association of Anaesthetists guidelines: cell salvage for peri-operative blood conservation 2018. Anaesthesia 2018;73:1141-50.
33. Girardi LN, Ohmes LB, Lau C, et al. Open repair of descending thoracic and thoracoabdominal aortic aneurysms in patients with preoperative renal failure. Eur J Cardiothorac Surg 2017;51:971-7.
34. Schepens MA, Heijmen RH, Ranschaert W, Sonker U, Morshuis WJ. Thoracoabdominal aortic aneurysm repair: results of conventional open surgery. Eur J Vasc Endovasc Surg 2009;37:640-5.
35. Estrera AL, Sandhu HK, Charlton-Ouw KM, et al. A quarter century of organ protection in open thoracoabdominal repair. Ann Surg 2015;262:660-8.
36. Chatterjee S, Shake JG, Arora RC, et al. Handoffs from the operating room to the intensive care unit after cardiothoracic surgery: from the Society of Thoracic Surgeons Workforce on Critical Care. Ann Thorac Surg 2019;107:619-30.
37. Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993;17:357-68; discussion 368.
38. Chatterjee S, Preventza O, Orozco-Sevilla V, Coselli JS. Critical care management after open thoracoabdominal aortic aneurysm repair. J Cardiovasc Surg (Torino) 2021;62:220-9.
39. Tanaka A, Safi HJ, Estrera AL. Current strategies of spinal cord protection during thoracoabdominal aortic surgery. Gen Thorac Cardiovasc Surg 2018;66:307-14.
40. Youngblood SC, Tolpin DA, LeMaire SA, Coselli JS, Lee VV, Cooper JR, Jr. Complications of cerebrospinal fluid drainage after thoracic aortic surgery: a review of 504 patients over 5 years. J Thorac Cardiovasc Surg 2013;146:166-71.
41. Weaver KD, Wiseman DB, Farber M, Ewend MG, Marston W, Keagy BA. Complications of lumbar drainage after thoracoabdominal aortic aneurysm repair. J Vasc Surg 2001;34:623-7.
42. Wynn MM, Mell MW, Tefera G, Hoch JR, Acher CW. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: a report of 486 patients treated from 1987 to 2008. J Vasc Surg 2009;49:29-34; discussion 34.
43. Chen DW, Price MD, LeMaire SA, Coselli JS, Liou NE, Ongkasuwan J. Early versus late inpatient awake transcervical injection laryngoplasty after thoracic aortic repair. Laryngoscope 2018;128:144-7.
44. Songdechakraiwut T, Aftab M, Chatterjee S, et al. Tracheostomy after thoracoabdominal aortic aneurysm repair: risk factors and outcomes. Ann Thorac Surg 2019;108:778-84.
45. Chatterjee S, Mulvoy W, Preventza O, de la Cruz KI, LeMaire SA, Coselli JS. ECMO for acute respiratory distress syndrome after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2018;106:e171-e2.
46. Dolapoglu A, Volguina IV, Price MD, Green SY, Coselli JS, LeMaire SA. Cardiac arrhythmia after open thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2017;104:854-60.
47. Maniar HS, Sundt TM, 3rd, Prasad SM, et al. Delayed paraplegia after thoracic and thoracoabdominal aneurysm repair: a continuing risk. Ann Thorac Surg 2003;75:113-9; discussions 119.
48. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84.
49. Frankel WC, Green SY, Amarasekara HS, et al. Early gastrointestinal complications after open thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2021;112:717-24.
50. Rubenstein C, Bietz G, Davenport DL, Winkler M, Endean ED. Abdominal compartment syndrome associated with endovascular and open repair of ruptured abdominal aortic aneurysms. J Vasc Surg 2015;61:648-54.
51. Chatterjee S, LeMaire SA, Green SY, et al. Is incidental splenectomy during thoracoabdominal aortic aneurysm repair associated with reduced survival? J Thorac Cardiovasc Surg 2020;160:641-52.e2.
52. Rahe-Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg 2009;138:694-702.
53. Cheng D, Martin J, Shennib H, et al. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease: a systematic review and meta-analysis of comparative studies. J Am Coll Cardiol 2010;55:986-1001.
54. Chatterjee S, Preventza O, Orozco-Sevilla V, Coselli JS. Perioperative management of patients undergoing thoracic endovascular repair. Ann Cardiothorac Surg 2021;10:768-77.
55. Daye D, Walker TG. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management. Cardiovasc Diagn Ther 2018;8:S138-S56.
56. Feezor RJ, Martin TD, Hess PJ, Jr. , et al. Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Ann Thorac Surg 2008;86:1809-14; discussion 1814.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Katz A, Suero OR, Orozco-Sevilla V, Preventza O, Moon MR, Coselli JS, Chatterjee S. Optimal perioperative care for thoracoabdominal and descending thoracic aortic aneurysm repair: a review. Vessel Plus 2023;7:1. http://dx.doi.org/10.20517/2574-1209.2022.52
AMA Style
Katz A, Suero OR, Orozco-Sevilla V, Preventza O, Moon MR, Coselli JS, Chatterjee S. Optimal perioperative care for thoracoabdominal and descending thoracic aortic aneurysm repair: a review. Vessel Plus. 2023; 7: 1. http://dx.doi.org/10.20517/2574-1209.2022.52
Chicago/Turabian Style
Katz, Abraham, Orlando R. Suero, Vicente Orozco-Sevilla, Ourania Preventza, Marc R. Moon, Joseph S. Coselli, Subhasis Chatterjee. 2023. "Optimal perioperative care for thoracoabdominal and descending thoracic aortic aneurysm repair: a review" Vessel Plus. 7: 1. http://dx.doi.org/10.20517/2574-1209.2022.52
ACS Style
Katz, A.; Suero OR.; Orozco-Sevilla V.; Preventza O.; Moon MR.; Coselli JS.; Chatterjee S. Optimal perioperative care for thoracoabdominal and descending thoracic aortic aneurysm repair: a review. Vessel Plus. 2023, 7, 1. http://dx.doi.org/10.20517/2574-1209.2022.52
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 17 clicks
Cite This Article 17 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.