More efficient clinical trials in pancreatic cancer: develop better treatment options, faster
Abstract
Clinical development of new treatment options for patients with pancreatic cancer has been slow and expensive and resulted in few effective therapies. With a dismal five-year survival rate of 11% in the U.S., pancreatic cancer remains the third leading cause of cancer-related deaths and is poised to move to second by 2030. Standard clinical trials typically compare one investigational treatment to one standard of care, encompass one phase of clinical investigation at a time, and treat one patient population. Accrual and data analysis are often very slow, and unfortunately, the vast majority of clinical trials targeting pancreatic cancer patients are unsuccessful. More efficient clinical trial designs can include combining phases I and II or phases II and III, and trials that involve a master protocol approach can also answer multiple clinical questions simultaneously. These modern clinical trial designs can allow a faster, more efficient and cost-effective approach to testing investigational therapies in patients with pancreatic cancer and, most importantly, fewer patients may be required to determine the efficacy of treatment. Herein we summarize some of the recent innovative clinical trials in pancreatic cancer to provide meaningful data toward developing new treatment options to benefit patients with a dismal disease like pancreatic cancer.
Keywords
INTRODUCTION
Pancreatic cancer is notable as one of the deadliest cancers. In 2020, it caused almost as many deaths (466,000) as cases (496,000)[1] worldwide. Currently, incidence and mortality rates are increasing in the U.S. and many other highly developed nations[1,2], leading to the projection that pancreatic cancer will overtake colorectal cancer to become the second leading cause of cancer deaths in the U.S. by 2030[3]. The difference between the two is that among the cancers tracked in the U.S., pancreatic cancer has the lowest five-year relative survival rate of 11%[2].
The reasons for the poor prognosis of patients diagnosed with pancreatic adenocarcinoma are expected and remain the same: too few patients are diagnosed when the disease is in the early, surgically resectable stage, and too few treatment options provide durable responses. The biological underpinnings of the challenges in treating pancreatic cancer include mutant KRAS as the driver gene in > 90% of cases, a pathway that has yet to be effectively targeted, a dense fibrotic stroma that limits the uptake of nutrients, oxygen and chemotherapies, an exceptionally immunosuppressive microenvironment, and an ability to metastasize at an early stage before symptoms are apparent[4]. In the search for new, better treatment options, the standard clinical trials process has failed many pancreatic adenocarcinoma patients - historically, trials have been designed with poor alignment with real-world patient characteristics, and too many trials have progressed to later stages without sufficient clinical evidence of their potential for success[5-7]. Oncology drug development is estimated to take more than seven years overall, costing an average of $37.8 M for each drug tested in phase I-III trials[8]. Despite the high barriers to clinical trial enrollment, thousands of pancreatic cancer patients participated in pivotal phase III trials over the past few years, with only 11% of trials resulting in clinically meaningful changes, and most patients learning that the investigational treatment did not provide a survival advantage over the standard of care[5]. The subsets of patients for whom the therapy did show benefit are infrequently studied to understand whether conditions apply for which this treatment may be appropriate to administer.
The lack of progress is not from a lack of trying. Since 2019, more than 125 pancreatic cancer clinical trials have been opened in the U.S. each year[9]. The trends over time indicate an increasing number of trials for previously treated patients, the emergence of trials for post-adjuvant or maintenance therapy, and increases in the number of research-intensive phase 0 trials and the number of phase III trials despite historical failures. Additionally, there was a dramatic increase in immunotherapy trials over this time and several biomarker-specified trials were initiated, indicating an increase in a precision medicine approach to pancreatic cancer treatment. Also notable was a small increase in the number of seamless phase I/II and II/III trials, an approach that is viewed as an attempt to improve the efficiency of the trial process.
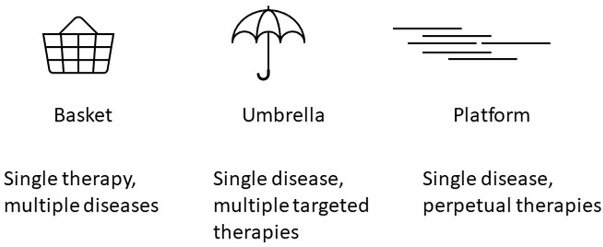
The U.S. Food and Drug Administration (FDA) has acknowledged that more novel clinical trial designs can be attractive to overcome some of the challenges identified in pancreatic cancer clinical research[10] [Figure 1]. For example, when tumor alterations may be rare within a single tumor type but may occur across multiple tumors, a basket trial is an excellent avenue that has led to the tumor-agnostic approvals of Pembrolizumab and other therapies that can be applied to occasional pancreatic cancer patients[11]. Focusing on a single cancer type at a time, umbrella trials allow patients to be assigned personalized arms of treatment based on their and their tumor’s biological and clinical characteristics[10]. Beyond data on the efficacy of platinum-based chemotherapies and PARP inhibition in pancreatic cancer patients with germline and/or somatic DNA damage repair alterations[12], there are few molecularly matched treatments offered to patients as standard practice. Umbrella clinical trials can provide additional data necessary for standardizing these approaches.
Figure 1. Novel clinical trial designs include basket, umbrella, and platform trials. Adapted from[10].
A platform clinical trial design allows multiple investigational treatment options to be tested in parallel against a single or several standard-of-care arms[10]. With constant evaluation of data and careful statistical analyses, it can quickly be determined whether a treatment arm is showing promise or faring poorly. Treatment arms can go on or off the platform, potentially allowing the trial to go on in perpetuity. Platform trials can also be designed to straddle two phases - phase I and II or phase II and III - to facilitate a smoother transition between dose escalation and dose expansion or to move more quickly from a positive efficacy signal to registration for approval.
With the poor clinical outcomes of pancreatic cancer and the lack of responsiveness to conventional and newer (e.g., immunotherapy) solid tumor treatment options, it is time to develop new approaches to combat this deadly cancer, as well as new ways to identify, develop and test new treatment options. Here in this opinion paper, we discuss the current standard of care for pancreatic adenocarcinoma in the U.S. and some recent innovations in clinical trials for pancreatic cancer, which provide hope for accelerated progress in the near future.
APPROVED TREATMENTS FOR PANCREATIC ADENOCARCINOMA IN THE UNITED STATES
Advances in the treatment of pancreatic adenocarcinoma in the U.S. depend primarily on clinical research leading to FDA approval for new treatment entities, although successful clinical trials with agents that are off-patent have also changed the standard. Progress was slow between 1995 and 2015; gemcitabine became the standard of care for metastatic pancreatic adenocarcinoma in 1996[13], FOLFIRINOX was added in 2010[14], and the combination of gemcitabine and nab-paclitaxel was FDA approved in 2013[15] [Table 1]. However, since 2015 there has been a remarkable increase in new treatments for specific disease states or sub-populations of patients. With FOLFIRINOX and gemcitabine plus nab-paclitaxel both considered effective standard-of-care options for metastatic patients, research efforts resulted in the first FDA approval of second-line treatment of 5-FU + nal-irinotecan for metastatic pancreatic adenocarcinoma patients who received gemcitabine in the first line[16]. There have also been accepted clinical advances resulting in the widespread use of gemcitabine-based or FOLFIRINOX-based regimens for adjuvant treatment for earlier-stage pancreatic adenocarcinoma[17,18]. Additionally, a number of approvals for biomarker-identified subsets of patients in tumor-agnostic studies impact a small number of pancreatic adenocarcinoma patients with NTRK fusion[19,20], germline BRCA1/2 or PALB2 mutations[12], or microsatellite instability, deficient mismatch repair, or high tumor mutational burden status[21-23]. Most recently, the combination of Dabrafenib-Trametinib was approved for solid tumors with BRAFv600E mutations partly because of the innovative NCI-MATCH basket trial[24]. Real-world data confirm the benefit of this combination in pancreatic cancer patients with this molecular alteration[25]. Although the numbers may be modest, these advances have a significant impact on the lives of the patients eligible for these treatments and a remarkable increase in the rate with which clinical advances are being applied to the treatment of this complicated disease.
Approved treatments for pancreatic adenocarcinoma in the U.S.
| Year | Treatment | Population | Approval | Reference |
| 1996 | Gemcitabine | Metastatic, 1st line | FDA | [13] |
| 2005 | Gemcitabine + erlotinib | Metastatic, 1st line | FDA | [36,37] |
| 2010 | FOLFIRINOX | Metastatic, 1st line | [14] | |
| 2013 | Gemcitabine + nab-paclitaxel | Metastatic, 1st line | FDA | [15] |
| 2015 | 5-FU + nal-irinotecan | Metastatic, post gemcitabine | FDA | [16] |
| 2016 | Gemcitabine + capecitabine | Post-surgery adjuvant | [17] | |
| 2017 | Pembrolizumab | Microsatellite instability (MSI-Hi) or deficient mismatch repair (dMMR) (approx. 2% prevalence) | FDA tissue agnostic | [22] |
| 2018 | Modified FOLFIRINOX | Post-surgery adjuvant | [18] | |
| 2018 | Larotrectinib | NTRK fusions, refractory (approx. 1% prevalence) | FDA, tissue agnostic | [19] |
| 2019 | Entrectinib | NTRK fusions, refractory (approx. 1% prevalence) | FDA, tissue agnostic | [20] |
| 2019 | Olaparib | Germline BRCA1/2, maintenance (approx. 5% prevalence) | FDA | [12] |
| 2020 | Pembrolizumab | High tumor mutation burden (TMB) (approx. 1% prevalence[38] | FDA, tissue agnostic | [21] |
| 2021 | Dostarlimab-gxly | Mismatch repair deficiency (dMMR) (approx. 2% prevalence) | FDA, tissue agnostic | [23] |
| 2022 | Dabrafenib plus trametinib | BRAFv600E (less than 1% prevalence)[25] | FDA, tissue agnostic | [24] |
MODERN CLINICAL TRIAL DESIGNS FOR PATIENTS WITH PANCREATIC CANCER
The momentum in pancreatic cancer clinical trial advances has energized the field and resulted in a number of innovative and exciting clinical trial designs focused on accelerating progress. These trials represent a multi-institutional collaborative effort among many investigators who have gathered forces to tackle the daunting problem of pancreatic adenocarcinoma. These efforts are summarized below and indicated by their focus on precision medicine, immunotherapy, or general platform trials.
1. Precision medicine
Precision medicine in pancreatic cancer has been advanced by organizations/projects with large tissue collections, such as the International Cancer Genome Consortium[26] and the Cancer Genome Atlas[27], as well as real-world efforts, such as the Pancreatic Cancer Action Network’s Know Your Tumor® program[28,29]. To link high-quality DNA and RNA sequencing with patient outcomes, the prospective Comprehensive Molecular Characterization of Advanced Pancreatic Ductal Adenocarcinoma for Better Treatment Selection (COMPASS, NCT02750657) trial was initiated in 2015 at several institutions in Canada, including Princess Margaret Cancer Center, Kingston Health Sciences Centre, Universite de Montreal, and McGill University. This observational study concluded that prospective genomic profiling of advanced pancreatic adenocarcinoma is feasible and provides a platform to better understand chemotherapy response among patients with different genomic/transcriptomic subtypes[30,31].
Precision-Panc (NCT04161417) was founded in 2017 in the U.K., bringing together the expertise of several Universities, Cancer Research UK research institutes, and the National Health Service[32]. A master protocol provides extensive molecular profiling and is a “portal” to sub-studies referred to as PRIMUS (Pancreatic canceR Individualised Multi-arm Umbrella Study). Precision-Panc enrolls patients with diagnosed or suspected pancreatic adenocarcinoma who undergo a biopsy for molecular profiling to determine their eligibility and enrollment in a PRIMUS substudy. The various PRIMUS studies allow for the enrollment of patients with different stages of pancreatic adenocarcinoma and look for predictive biomarkers to standard-of-care chemotherapy as well as targeted agents, immunotherapies and other investigational drugs.
2. Immunotherapy platforms
REVOLUTION (NCT04787991), an exploratory platform in the phase I setting sponsored by the Parker Institute for Cancer Immunotherapy and the Cancer Research Institute, opened in 2021 at seven sites within the U.S. REVOLUTION enrolls treatment-naïve patients with metastatic pancreatic adenocarcinoma and tests combination approaches that include immunotherapy. An exploratory platform, REVOLUTION can add and drop investigational arms based on results. The two-stage design allows for early assessment of predictive biomarkers, safety and efficacy, followed by an expanded second stage to confirm the earlier-stage observations.
Morpheus-Pancreatic Cancer, a Study of Multiple Immunotherapy-Based Treatment Combinations in Participants with Metastatic Pancreatic Ductal Adenocarcinoma (NCT03193190), is a phase Ib/II clinical trial sponsored by Hoffmann-La Roche that opened at 27 study locations in 2017. Morpheus-Pancreatic Cancer evaluates multiple immunotherapeutic approaches for the treatment of patients with metastatic pancreatic adenocarcinoma. Who have received no prior treatment or have received one treatment regimen. The platform nature of this trial allows new investigational arms to be added as preclinical and clinical evidence suggests their potential efficacy, and arms will be removed from the study due to futility.
3. Adaptive platform trials
Trials using adaptive designs are gaining traction in the pancreatic cancer field after their success in other cancer types. The breast cancer I-SPY 2 study (Investigation of Serial Studies to Predict Your Therapeutic Sesponse with Imaging and Molecular Analysis 2 - NCT01042379) was launched in 2011 and continues to enroll patients and set examples for adaptive platform trials in cancer[33]. The GBM AGILE (Glioblastoma Adaptive Global Innovative Learning Environment - NCT03970447) adaptive platform clinical trial is testing experimental therapies for patients with glioblastoma and brought several innovations to the platform trial design[34]. Based on the response-adaptive nature of the trial, investigational treatments can progress through the trial based on their efficacy in either/both first-line patients or those experiencing a recurrence, and therapeutic arms will drop from the trial if not found to be effective in either patient population. The GBM AGILE trial is designed to be conducted in two stages: the screening stage is intended to identify investigational treatments that show benefit over standard-of-care control arms and to identify the patient population(s) for which the arm is efficacious; then, the confirmatory stage involves fixed randomization to validate the findings in the patient population(s) identified through the screening phase and prepare the treatment for registration for the approval process. Lessons learned from the design and execution of GBM AGILE can inform future clinical trials in pancreatic cancer, as the two diseases have similarly low incidence rates and extremely poor survival rates.
Precision PromiseSM (NCT04229004), sponsored by the Pancreatic Cancer Action Network (PanCAN), opened to recruitment in 2020 at 12 sites and is currently expanding to 30 sites. It is a phase II/III responsive adaptive clinical trial for patients with metastatic pancreatic adenocarcinoma in the first or second line of treatment. Like GBM-AGILE and I-SPY 2, the Bayesian adaptive statistical design of Precision Promise is powered by Berry Consultants. In addition to screening and confirmatory trial stages to allow for the development of a registration packet, this trial adds a feature of re-randomization to eligible patients who enrolled in Precision Promise prior to treatment and whose first-line treatment within the trial is unsuccessful. Precision Promise is designed to continuously evaluate multiple experimental arms (70%) vs. two control arms (15% gemcitabine+nab-paclitaxel or 15% mFOLFIRINOX) in patients with metastatic pancreatic adenocarcinoma. The primary endpoint is overall survival. Compared to traditional trial designs, Precision Promise offers several advantages: multiple investigational treatments can be evaluated in parallel over time; only ~175 patients per experimental arm are required to initiate a regulatory registration; and increased learning from every patient during the trial, altogether resulting in both time-saving and a 30%-50% cost saving. In addition to evaluating the efficacy of investigational treatments, Precision Promise incorporates innovative supportive care measures like actigraphy to prioritize patients’ quality of life and includes additional clinical parameters to test within the trial. In effect, Precision Promise has created an entirely new “learning community” and can substantially accelerate drug development for pancreatic adenocarcinoma.
Pioneer-Panc (NCT04481204) is a platform trial for randomized phase II investigations in localized pancreatic adenocarcinoma that is soon to be underway[35]. Using a Bayesian platform design to evaluate multiple experimental arms against a control arm, Pioneer-Panc focuses on patients with resectable, borderline resectable, and locally advanced disease and further divides each stage group based on treatment history (treatment naïve or previously treated). Within each cohort, adaptive randomization rules are applied and patients will be randomized to either an experimental arm or the control arm accordingly. Experimental arms may be added independently to one or more cohorts during the study, and multiple correlative studies for tissue, blood, and imaging are incorporated.
CONCLUSION AND FUTURE DIRECTIONS
There is considerable momentum in the pancreatic cancer field after a long period during which there were minimal advances in the standard of care. As Table 1 shows, there were five advances in the standard of care for pancreatic adenocarcinoma in the 20 years between 1995 and 2015, whereas nine approvals or advances were recorded in the seven years between 2015 and 2022, albeit for a small number of patients. The field is galvanized for even greater acceleration of progress over the next decade by the addition of several innovative trials that gather and focus the expertise of many investigators on the recalcitrant problem of pancreatic cancer treatment. These studies range from observational studies such as COMPASS that seek to better understand how the molecular underpinnings of pancreatic adenocarcinoma impact treatment, to phase I and Ib/II signal-seeking trials such as REVOLUTION and Morpheus with a focus on immunotherapy development, to the highly flexible Precision-Panc master protocol that allows the addition of PRIMUS sub-studies for many different sub-populations of pancreatic adenocarcinoma, to the phase II Pioneer-Panc focused on localized pancreatic adenocarcinoma and the ambitious Precision Promise phase II/III trial designed to de-risk and accelerate progress for FDA approval for new standards of care for metastatic pancreatic adenocarcinoma. The field has embraced the Master Protocol concept[10] and banded together to rise to the challenge. There is considerable optimism that the next decade of clinical research will yield the advances pancreatic cancer patients desperately need and deserve.
DECLARATIONS
AcknowledgmentsThe authors wish to acknowledge and thank all pancreatic cancer patients who contribute to clinical research and provide hope to those patients of the future.
Authors’ contributionsContributed to the writing: Rosenzweig A, Matrisian LM
Contributed to the review of this manuscript: Rosenzweig A, Moravek C, Matrisian LM
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the Pancreatic Cancer Action Network.
Conflicts of interestAll authors are employees of the Pancreatic Cancer Action Network and have no personal conflicts of interest with any commercial interests mentioned in this publication. The Pancreatic Cancer Action Network has received donations from the following companies over the past 2 years: AbbVie, Amgen Foundation, AstraZeneca Pharmaceuticals, Boston Scientific, Bristol-Myers Squibb, Corcept, Covance, Elevation Oncology, Eli Lilly, Fibrogen, Fujifilm Pharmaceuticals USA, Genentech, GlaxoSmithKline, GRAIL, Immunovia, Ipsen Biopharmaceuticals, Johnson & Johnson, Merck Sharpe & Dohme Corp, Mirati Therapeutics, Novocure, Novartis Pharmaceuticals, Pfizer, Rafael Pharmaceuticals, Servier Pharmaceuticals, Takeda Pharmaceuticals NA, Tempus Health, TriSalus Life Sciences, Tyme, View Ray. Berry Consultants are vendors for the Precision Promise clinical trial.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49.
3. Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open 2021;4:e214708.
4. Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol 2020;17:108-23.
5. Hoos WA, James PM, Rahib L, Talley AW, Fleshman JM, Matrisian LM. Pancreatic cancer clinical trials and accrual in the United States. J Clin Oncol 2013;31:3432-8.
6. Rahib L, Fleshman JM, Matrisian LM, Berlin JD. Evaluation of pancreatic cancer clinical trials and benchmarks for clinically meaningful future trials: a systematic review. JAMA Oncol 2016;2:1209-16.
7. Matrisian LM, Berlin JD. The past, present, and future of pancreatic cancer clinical trials. Am Soc Clin Oncol Educ Book 2016;35:e205-15.
8. Sertkaya A, Birkenbach A, Berlind A, Eyraud J. Examination of clinical trial costs and barriers for drug development, In: Report HHSP23337007T, Department of Health and Human Services (HHS): Washington USA. 2014.
9. Matrisian LM, Rosenzweig A, Moravek C, Duliege AM. Trends in pancreatic cancer clinical trials in the United States. Med Res Arch 2021; doi: 10.18103/mra.v9i11.2578.
10. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med 2017;377:62-70.
11. Seligson ND, Knepper TC, Ragg S, Walko CM. Developing drugs for tissue-agnostic indications: a paradigm shift in leveraging cancer biology for precision medicine. Clin Pharmacol Ther 2021;109:334-42.
12. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317-27.
13. Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13.
14. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25.
15. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703.
16. Wang-gillam A, Li C, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57.
17. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24.
18. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395-406.
19. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018;378:731-9.
20. Doebele RC, Drilon A, Paz-ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82.
21. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353-65.
22. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13.
23. FDA grants accelerated approval to dostarlimab-gxly for dMMR advanced solid tumors, 2021. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors [Last accessed on 13 Oct 2022].
24. Salama AKS, Li S, Macrae ER, et al. Dabrafenib and trametinib in patients with tumors with BRAFV600E mutations: results of the NCI-MATCH trial subprotocol H. J Clin Oncol 2020;38:3895-904.
25. Hendifar A, Blais EM, Wolpin B, et al. Retrospective case series analysis of RAF family alterations in pancreatic cancer: real-world outcomes from targeted and standard therapies. JCO Precis Oncol 2021;5:PO.
26. Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501.
27. Genome Atlas Research Network. Electronic address, Cancer genome atlas research network. integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 2017;32:185-203.e13.
28. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol 2020;21:508-18.
29. Pishvaian MJ, Bender RJ, Halverson D, et al. Molecular profiling of patients with pancreatic cancer: initial results from the know your tumor initiative. Clin Cancer Res 2018;24:5018-27.
30. Aung KL, Fischer SE, Denroche RE, et al. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin Cancer Res 2018;24:1344-54.
31. Chan-Seng-Yue M, Kim JC, Wilson GW, et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat Genet 2020;52:231-40.
32. Dreyer SB, Jamieson NB, Cooke SL, et al. PRECISION-Panc: the next generation therapeutic development platform for pancreatic cancer. Clin Oncol 2020;32:1-4.
33. Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 2009;86:97-100.
34. Alexander BM, Ba S, Berger MS, et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res 2018;24:737-43.
35. Douglas JE, Liu S, Ma J, et al. PIONEER-panc: a platform trial for phase II randomized investigations of new and emerging therapies for localized pancreatic cancer. BMC Cancer 2022;22:14.
36. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6.
37. Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27:2231-7.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Rosenzweig A, Moravek C, Matrisian LM. More efficient clinical trials in pancreatic cancer: develop better treatment options, faster. J Cancer Metastasis Treat 2022;8:46. http://dx.doi.org/10.20517/2394-4722.2022.58
AMA Style
Rosenzweig A, Moravek C, Matrisian LM. More efficient clinical trials in pancreatic cancer: develop better treatment options, faster. Journal of Cancer Metastasis and Treatment. 2022; 8: 46. http://dx.doi.org/10.20517/2394-4722.2022.58
Chicago/Turabian Style
Rosenzweig, Allison, Cassadie Moravek, Lynn M. Matrisian. 2022. "More efficient clinical trials in pancreatic cancer: develop better treatment options, faster" Journal of Cancer Metastasis and Treatment. 8: 46. http://dx.doi.org/10.20517/2394-4722.2022.58
ACS Style
Rosenzweig, A.; Moravek C.; Matrisian LM. More efficient clinical trials in pancreatic cancer: develop better treatment options, faster. J. Cancer. Metastasis. Treat. 2022, 8, 46. http://dx.doi.org/10.20517/2394-4722.2022.58
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 23 clicks
Cite This Article 23 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.