Clinical translation of genetic testing in TTR Amyloidosis: genotype-phenotype correlations, management of asymptomatic carriers and familial screening
Abstract
Transthyretin (TTR)-related amyloidosis (ATTR) is a heterogeneous disease with different organ involvement depending on the type of TTR infiltration [mutated (vTTR) or wild-type (wtTTR)]. Genetic testing in ATTR is required to define diagnosis and identify asymptomatic at-risk family members. Since new therapies are maximally effective in the early stages of the disease, there is a growing agreement about the need for close monitoring of genotype-positive, phenotype-negative individuals to assure a prompt treatment when minor disease signs are detected. This review summarizes the complexity of genotype-phenotype correlation and revises the current indications with respect to familiar screening and management of asymptomatic carriers.
Keywords
INTRODUCTION
Transthyretin (TTR)-related amyloidosis (ATTR) is caused by extracellular deposition of the misfolded TTR protein in different tissues, particularly in the heart and the autonomic and peripheral nerve fibers. TTR infiltration in the myocardium causes infiltrative cardiomyopathy characterized by progressive diastolic and systolic dysfunction, leading to heart failure[1]. The dissociation of the TTR tetramer into monomers, followed by misfolding and assembling in amyloid fibrils, may be promoted by a single point mutation in the TTR gene (hereditary, ATTRv) or by aging (wild type, ATTRwt)[1]. The supposed mechanisms of the latter form are still largely unknown; some aging-related processes are probably involved in protein destabilization, considering that all protein-misfolding diseases have age-related penetrance. ATTRv is an autosomal-dominant disease associated with more than 100 different pathogenic mutations in the TTR gene[2]. The diverse mutations are associated with extremely heterogeneous clinical manifestations ranging from a predominant cardiac involvement to an exclusively neurological one, with a variety of mixed phenotypes[1,3]. There are no demonstrated correlations among gene localization of the different TTR mutations, protein dysfunction, and clinical phenotype. Amyloid fibrils may contain two different types of fibrils: Type A, composed of amixture of C-terminal fragments and full-length TTR, and Type B, formed only by full-length TTR. Type A fibrils are more frequent, while Type B fibrils, up to date, are an exception and have been found in a limited number of TTR mutations, such as Phe64Leu and Val30Met[4,5].
While ATTRv is still considered a rare condition, ATTRwt cardiac amyloidosis is now increasingly recognized and represents one of the most important causes of heart failure with preserved ejection fraction (HFpEF); in fact, TTR deposits can be seen in up to 30% of older adults with HFpEF who undergo autopsy[6].
The recent meaningful increase of identified cases over the past five years is the result of the growing awareness of the disease, together with the availability of non-invasive diagnostic tests such as bone scintigraphy[7]. Moreover, the advent of new drugs, which seem to be able to slow down the amyloidogenic process, has revolutionized the clinical approach to this disease, especially because the effectiveness of the new therapies is maximum in the early stages[8].
Clinical spectrum of ATTRv
In contrast to ATTRwt, in which the infiltration is limited to the heart and soft tissues, ATTRv has a wider clinical spectrum, depending on the type of TTR mutation and the degree of organ involvement. Mainly, it affects the peripheral and autonomic nervous systems and/or the heart. On the basis of the extent of these two organs impairment, three phenotypes of ATTRv can be distinguished: neurological, cardiac, and mixed form[9]. In an Italian cohort, the vast majority of the affected patients had neurological involvement, while about two-thirds had cardiac involvement; a significant minority (15%) had an exclusive cardiac phenotype[10].
Neurological involvement consists largely in peripheral sensory-motor polyneuropathy, which firstly affects lower extremity small sensory fibers with early impairment of pain and temperature sensation. Autonomic neuropathy may dominate the phenotypic expression or be an early sign of the disease[11].
Cardiac involvement results in restrictive cardiomyopathy characterized by left and right ventricular hypertrophy and advanced diastolic dysfunction with preserved ejection fraction. Indeed, fibrils infiltration in the myocardium leads to increased wall thickness (pseudo-hypertrophy). Although heart failure usually dictates the clinical course of cardiac amyloidosis, amyloid infiltration causes a wide extent of myocardial derangement, which may result in several other clinical manifestations, including tachy- and bradyarrhythmias[3,9,12].
GENOTYPE-PHENOTYPE CORRELATION
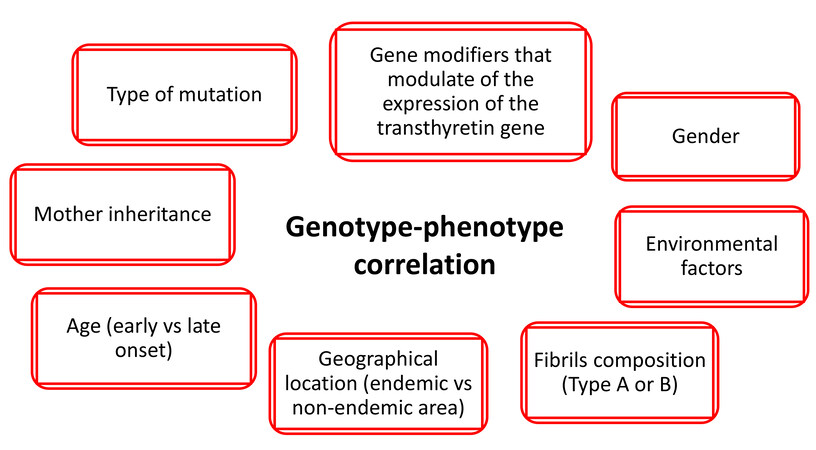
Phenotypic expression is mostly influenced by the causative TTR gene mutation. However, even in the presence of the same mutation, clinical manifestations may differ among individuals due to other determinants, e.g., age, gender, fibril composition, geographical location, environmental factors, and modulator genes[3,13] [Figure 1].
Taking into account the type of TTR gene mutation, ATTR can be divided into four main groups: Val30Met early onset (neurological), Val30Met late onset (neurological/mixed), non-Val30Met mixed phenotype, and non-Val30Met cardiac phenotype. Five main mutations are associated with an exclusive or predominant cardiac phenotype: Val122Ile, Thr60Ala, Val30Met (late-onset), Ile68Leu, and Leu111Met[3].
Most TTR mutations are linked to a specific geographical location, and each mutation presents a distinct phenotype, as described in [Table 1][14-29]. In a unique manner, the Italian scenario is characterized by high genotypic heterogeneity: 26 different mutations can be identified, and the most frequent are Val30Met, followed by Glu89Gln, Ile68Leu, and Phe64Leu[10].
Clinical features of the most commonTTR mutations
| Val30Met early onset | Val30Met late onset | Val122Ile | Ile68Leu | Glu89Gln | Phe64Leu | Thr60Ala | Leu111Met | Ile107Val | |
| Allele frequency | 0.19% | 1.5%-4% | 3%-4% in black Americans | Unknown | Unknown | Unknown | 1% | Unknown | Unknown |
| Inheritance | AD | AD | AD | AD | AD | AD | AD | AD | AD |
| Penetrance | High | Low | Low, higher in males | Low, higher in males | High | Medium | Low | High | Unknown |
| Age of onset | 30-40 years | 50-60 years | 70 years | 70 years | 50-60 years | 65-70 years | 50-60 years | 30-40 years | 65 years |
| Geographic location | Portugal, Japan | Portugal, Japan,Sweden | USA, West Africa | Central-northern Italy | South Italy, Bulgaria | South Italy | North-west Ireland, UK | Denmark | France |
| Gender | M = F | M > F | M > > F | M > > F | M = F | M > F | M > > F | M = F | M > > F |
| Phenotype | Mainly neurologic involvement with autonomic neuropathy and gastrointestinal manifestations, frequent conduction disturbances | Mixed, more common motor neuropathy and cardiac involvement then in early onset | Mainly cardiac involvement | Mainly cardiac involvement, frequent CTS and, if present, mild neurologic involvement | Mixed, usually neuropathy as presenting symptom | Mixed, with earlier periphericthan autonomic neurologic involvement | Predominantly cardiac and autonomic involvement, < 1/4 patients with peripheral neuropathy | Exclusive cardiac involvement | Mainly peripheric neurologic involvement, upper limb weakness and gait disorders |
| Progression of the disease | Progressive peripheral sensorimotor and autonomic polyneuropathy, need of PMK | Walking disability, HF | HF, slow disease progression | HF, AF development, need of PMK for advanced AV block | Early heart dysfunction, cardiomyopathy as major cause of mortality followed by dysautonomia and cachexia | Severe peripheral neuropathy, death for wasting syndrome | HF, AF, need of PMK, progression of neuropathy. Poor prognosis | HF | Rapid onset of gait disturbances,tetraparesis, short median survival. |
| Peculiarities | Progression of neuropathy successfully halted by OLT | Severe and fast-progressing disease | Prognosis, clinical, ECG and echo features similar to ATTRwt | Low sensitivity of bone scintigraphy to detect CA | Disease progression not modified by OLT | High penetrance and early onset in contrast to other cardiac mutations | Most debilitating and severe neuropathy ever described |
Val30Met is probably the most common TTR mutation worldwide, particularly in Portugal, where it has an estimated prevalence of 0.09%, reaching 73.3% of detected mutations in the THAOS registry[15].It has variable disease manifestations and penetrance depending on geographic location[3,14-17]. The prominent features of Portuguese and Japanese endemic Val30Met ATTR are sensorimotor polyneuropathy and autonomic neuropathy, with early onset (third to fourth decade of life); cardiomyopathy is rare, while conduction disturbances are relatively frequent[15-18]. The clinical profile of Swedish endemic Val30Met ATTR is characterized by a slower disease progression with higher age of onset of disease (fifth to sixth decade of life) and lower penetrance[15-18]. Non-endemic Val30Met ATTR has a lower age-related penetrance with more frequent cardiomyopathy and milder autonomic dysfunction than endemic ones[15-18].
About 4% of African Americans are carriers of the Val122Ile TTR mutation. The predominant phenotypic feature of this mutation is severe restrictive cardiomyopathy with late onset (generally in the sixth or seventh decade of life) without neurologic involvement[17].
In some Italian regions, mainly Emilia-Romagna and Tuscany, the Ile68Leu TTR mutation is endemic, being responsible for the majority of cases with predominant cardiac involvement in this area, and it shares with Val122Ile advanced age at presentation, high prevalence of carpal tunnel syndrome (about 40% of patients affected), and a homogeneous echocardiographic profile with symmetric hypertrophy and a normal or near-normal left ventricle ejection fraction[19,20], resembling ATTRwt. Therefore, it is essential to perform genetic testing in all patients presenting with a phenotype consistent with ATTR cardiac amyloidosis, as outlined below.
Fibril composition can deeply influence the clinical presentation; indeed, in Val30Met ATTR, full-length TTR (Type B fibrils) is associated with earlier-onset disease with minor cardiac involvement, while fragmented TTR (Type A fibrils) is associated with later-onset disease with preeminent cardiac involvement[30]. Among Val30Met patients[4], it was noted that fibril composition was quite consistent between families suggesting that genetic, epigenetic, and environmental factors, all still unknown, may have a role in amyloid fibril composition. The fibril composition-clinical presentation correlation is still under evaluation; what has been described thus far is that patients with Type A fibrils after liver transplantation present heart failure worsening in contrast to patients with Type B fibrils[31] and that the majority of patients with Type A and no patient with Type B fibrils have myocardial uptake at bone scintigraphy[32]. The importance of amyloid fibril composition needs to be further largely evaluated to determine if it has prognostic and diagnostic implications.
Sex seems to have a protective role in ATTRv; data from the THAOS registry show that women who carry a pathogenic mutation, compared to men, less commonly have cardiac involvement (28% vs. 72%) with less severe manifestation[33]. Furthermore, the male prevalence was notably lower overall in asymptomatic carriers, suggesting a lower genetic penetrance in women compared with men[33]. In the Val122Ile mutation, there is also a sex-related genotype-phenotype expression correlation. Indeed, female Val122Ile patients present predominantly with polyneuropathy phenotype, while males have predominantly cardiovascular or mixed phenotype[16], and, among Val122Ile patients with cardiac involvement, females are significantly older at diagnosis compared to males (76 years vs. 69 years) even if they have similar cardiac chamber function and rate of mortality, suggesting a slower progression of disease in women[34]. All these differences are likely related to the interaction of epigenetic mechanisms and sex-related protective hormones, given that those women with higher degrees of myocardial involvement are more likely to be post-menopausal[35].
In addition, it has been reported that the age of onset in male offspring is earlier when the disease is inherited from the mother, leading to a higher penetrance[16,17]. This anticipation of disease onset may be caused by parental imprinting phenomenon, or it has been hypothesized to be a consequence of the interaction of the mitochondrial genome with the expression of the TTR gene[36].
Genetic variants located in the non-coding regions of the TTR gene can modulate the clinical phenotype influencing gene expression. Iorio et al.[37] demonstrated that specific expression patterns are related to particular phenotypic presentations, thus suggesting a key role of the differential TTR gene expression profiles across different tissues in determining the phenotype. In the same way, variants in the regulatory regions of the TTR gene may account for the large variability of the age of onset, as described for Val30Met patients[38,39].
When to perform the genetic test?
Genetic testing typically should be reserved for patients with a confirmed or suspected diagnosis of inherited disease and for asymptomatic at-risk family members[40]. Molecular confirmation of a diagnosis may help to avoid unnecessary testing and procedures, guide recommendations for medical treatment and screening, and offer accurate genetic counseling (including risk assessment) for the family. Similarly, in the evaluation of ATTR, genetic testing may be considered to confirm a suspected diagnosis of ATTRv and determine carrier status for a known familial mutation. Identification of patients whose amyloidosis is of genetic origin is of utmost importance, as it affects treatment decisions.
The diagnosis of cardiac ATTR is a challenge in daily clinical practice. According to Gillmore’s algorithm[6], in the presence of clinical suspicion of cardiac amyloidosis, it is mandatory to rule out a monoclonal gammopathy by means of three tests: serum-free light chains (sFLC), serum, and urine immunofixation electrophoresis. Moreover, a scintigraphy with bone avid tracers 99mtechnetium pyrophosphate (99mTc-PYP), 99mtechnetium 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), and 99mtechnetium hydroxymethylene diphosphonate (99mTc-HMDP) should be performed; if the results are positive, this allows making a cardiac-ATTR diagnosis without the need of an endomyocardial biopsy[6,41,42]. In particular, cardiac ATTR can be diagnosed when all of the following criteria are met: heart failure with an echocardiogram or cardiac magnetic resonance (CMR) that is suggestive of amyloidosis; grade 2 or 3 cardiac uptake on a radionuclide scan with 99mTc-PYP, 99mTc-DPD, or 99mTc-HMDP; and absence of a detectable monoclonal protein despite sFLC, serum, and urine immunofixation electrophoresis assay[6,7]. Histological confirmation is needed in all cases of suspected cardiac amyloidosis in which these criteria are not met[6,7]. The first cause of false positive scans is AL amyloidosis which should obligatorily be excluded. On the contrary, false negative scans may occur in the presence of some ATTRv mutations such as Phe64Leu that is characterized by low or absent myocardial bone tracer uptake; therefore, in such cases, in the presence of high clinical suspicion, a multimodal approach with CMR, cardiac biopsy, and/or genetic testing should be sought[43].
Once the diagnosis of cardiac amyloidosis has been made, genetic testing has to be performed in all patients, regardless of the presence of family history, in order to distinguish ATTRwt amyloidosis from the mutated form [7,44-46]. The genetic test has a fundamental role even if the proband is old, given that certain specific mutations are characterized by a late clinical onset[7,44-46].
Genetic testing of TTR mutations should also be offered to patients with neurological symptoms suggestive of ATTR: unexplained dysautonomia, progressive sensory length-dependent polyneuropathy, or rapidly progressive sensory-motor axonal neuropathy in the absence of other possible causes (such as diabetes, alcohol abuse, monoclonal gammopathy, vitamin B12 deficiency, or hypothyroidism)[47].
When a diagnosis of hereditary amyloidosis has been established and the mutation identified, genetic study of relatives can determine who needs to be monitored and who does not. In particular, genetic screening should be offered to the proband’s siblings, who are at higher risk for developing a clinical disease in the immediate future. Usually, the timing between the diagnosis of the index patient and the offer of genetic testing to its relatives should be flexible and should consider the specific mutation penetrance, as well as the age of onset and the severity of the disease both in the proband and in the other affected relatives[48,49]. Genetic studies in those under 18 years of age are not recommended, given that the result would not affect the clinical approach, while it could have possible negative psychological consequences for the minors and those around them[44,46]. Participation in genetic screening must be an autonomous personal choice, and long-term clinical monitoring can be offered to those who refuse to undergo genetic testing.
Doing the genetic test in asymptomatic relatives presents the potential psychological consequence of knowing to be at risk for developing a degenerative disease. Therefore, it should always be considered that genetic testing may be associated with the difficulty of accepting the results and their impact on professional life, family, and relationships[50]. Mutation carriers need to be aware of this, especially when the penetrance of the familial TTR mutation is low, as in Val30Met late-onset or non-Val30Met cardiac phenotype[51].
A recent study[52] highlighted the psychological consequences of the disease on both patients and at-risk relatives, since they may often feel depressed (60%) or worry about the future (75%-80%), as well as guilty of a possibility to pass the disease to offspring (40%-60%). Pre-symptomatic genetic testing can lead to anxiety, depression, avoidance of the disease, and psychological distress, even in the case of a negative result (“survivor guilt”), especially in women[53]. Therefore, psychological support has to be offered to the index patient and relatives, both to mutation carriers and those who did not undergo genetic testing or received a negative result[46,53].
Besides the strong psychological impact, being an asymptomatic gene carrier does not have consequences on daily life since there are no specific restrictions or particular recommendations regarding leisure and competitive sports or work activity. They should conduct themselves as other healthy people, except for regular neurological and cardiological examinations, as outlined below.
MANAGEMENT OF GENOTYPE-POSITIVE, PHENOTYPE-NEGATIVE INDIVIDUALS
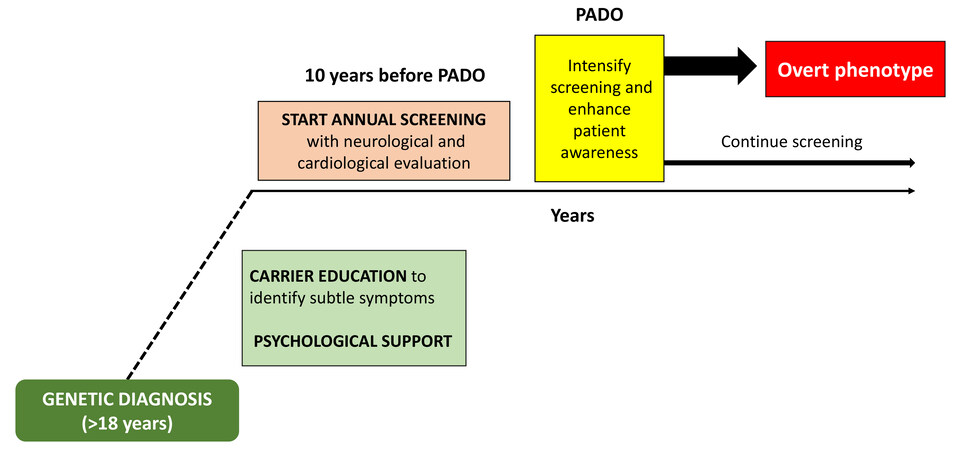
Since the new therapies are maximally effective in the early stages of the disease, there is a growing agreement about the need for close monitoring of genotype-positive, phenotype-negative individuals to assure a prompt treatment when minor disease signs are detected[44-46,48,54]. Recently, to determine when this monitoring should start, it has been proposed to estimate the predicted age of disease onset (PADO), which depends on the typical age of onset for the specific TTR gene mutation, the age of onset in other members of the proband’s family, and the sex of the parent who carries the mutated gene[54]. Expert consensus has recommended starting monitoring pre-symptomatic individuals 10 years before PADO[54,55]. Annual monitoring is the standard, but the frequency of screening visits can increase getting closer to the PADO, especially for genotypes associated with more rapid disease progression. Abnormal findings or symptoms could lead to a shorter follow-up to define their clinical significance[54,55]. Educating pre-symptomatic carriers to recognize early disease-related symptoms is of paramount importance.
Screening visits consist of neurological and cardiological evaluations. The tests carried out during the follow-up should be targeted at the expected phenotype for the specific mutation[55]. As regards the cardiological evaluation, 12-lead electrocardiogram (ECG), complete echocardiographic examination, and cardiac biomarkers measurement (troponin and natriuretic peptides) should be performed every year[7]. In addition, 24 hECG monitoring may be useful to be completed every two years[7]. CMR imaging and scintigraphy with bone tracers should be carried out every three years or if any of the above complementary tests is abnormal[7].
Bone scintigraphy is useful in the early identification of cardiac ATTR, even before wall thickness increases or electrocardiographic voltages reduction is observed[56,57]. Biopsies, derived from non-specific sites or clinically affected organs, are not routinely recommended due to their low sensitivity in the early stages so that a negative result does not permit ruling out the disease.
The neurological evaluation consists of searching for autonomic dysfunction and peripheral neuropathy[57]. Autonomic dysfunction may be investigated, looking for symptoms such as orthostatic hypotension, gastrointestinal disturbance, genitourinary signs, and erectile dysfunction, with the compound autonomic dysfunction test. Peripheral neuropathy should be searched with clinical scales such as neuropathy impairment score, nerve conduction studies to explore large fibers’ function, and different tools to evaluate small nerve fibers such as skin biopsy[54,58].
Figure 2 shows a simplified algorithm of management of ATTRv genotype positive, phenotype negative individuals.
Figure 2. Simplified algorithm of management of ATTRv genotype positive-phenotype negative individuals. PADO: Predicted age of disease onset.
Treatment initiation in gene carriers should start when the earliest detectable disease sign or symptom is found[49,59]. There is still no consensus that recommends initiation of disease-modifying therapy before detectable amyloid deposits or clinical signs/symptoms.
Asymptomatic gene carriers should be referred from peripheral centers to hub centers for amyloidosis in the absence of possibilities to perform diagnostic tests (i.e., lack of nuclear medicine department), if there is a doubt on clinical manifestations, to confirm or exclude them, or if hub centers are involved in clinical trials suitable for asymptomatic gene carriers.
CONCLUSIONS
ATTR is an increasingly recognized condition, but it is still commonly misdiagnosed. The diagnostic difficulties are at least partially caused by the wide phenotypic spectrum, even in the presence of the same mutation, and frequently cause a delay in diagnosis, which has an impact on survival. In the next few years, the growing awareness of the disease, together with the wider availability of non-invasive techniques and genetic testing, will lead to a greater knowledge of genotype-phenotype correlation, thus allowing a better understanding of disease incidence and penetrance and, hopefully, an earlier diagnosis and treatment.
The management of patients with a TTR mutation without an overt phenotype is challenging and crucial since new and effective disease-modifiers drugs, which may improve ATTRv natural history by reducing mortality, are available. The close monitoring of asymptomatic mutation carriers is necessary to promptly start specific therapy and maximize the benefits in terms of survival and quality of life.
DECLARATIONS
Authors’ contributionsWrote the entire manuscript: Scirpa R, Russo D
Essential support:Musumeci B
All authors reviewed the final manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Gertz MA, Benson MD, Dyck PJ, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol 2015;66:2451-66.
2. Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid 2003;10:160-84.
3. Rapezzi C, Lorenzini M, Longhi S, et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev 2015;20:117-24.
4. Suhr OB, Wixner J, Anan I, et al. Amyloid fibril composition within hereditary Val30Met (p. Val50Met) transthyretin amyloidosis families. PLoS One 2019;14:e0211983.
5. Ihse E, Rapezzi C, Merlini G, et al. Amyloid fibrils containing fragmented ATTR may be the standard fibril composition in ATTR amyloidosis. Amyloid 2013;20:142-50.
6. Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014;2:113-22.
7. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy Diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404-12.
8. Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC working group on myocardial and Pericardial diseases. Eur Heart J 2021;42:1554-68.
9. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2872-91.
10. Rapezzi C, Quarta CC, Obici L, et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J 2013;34:520-8.
11. Conceição I, González-Duarte A, Obici L, et al. “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst 2016;21:5-9.
12. Cappelli F, Tini G, Russo D, et al. Arterial thrombo-embolic events in cardiac amyloidosis: a look beyond atrial fibrillation. Amyloid 2021;28:12-8.
13. Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol 2010;7:398-408.
14. Damy T, Kristen AV, Suhr OB, et al. THAOS Investigators. Transthyretin cardiac amyloidosis in continental western europe: an insight through the transthyretin amyloidosis outcomes survey (THAOS). Eur Heart J ;2019:391-400.
15. Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin 2013;29:63-76.
16. Planté-Bordeneuve V, Suhr OB, Maurer MS, White B, Grogan DR, Coelho T. The Transthyretin amyloidosis outcomes survey (THAOS) registry: design and methodology. Curr Med Res Opin 2013;29:77-84.
17. Maurer MS, Hanna M, Grogan M, et al. THAOS Investigators. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin amyloid outcome survey). J Am Coll Cardiol 2016;68:161-72.
18. Conceição I, De Carvalho M. Clinical variability in type I familial amyloid polyneuropathy (Val30Met): comparison between late- and early-onset cases in Portugal. Muscle Nerve 2007;35:116-8.
19. Gagliardi C, Perfetto F, Lorenzini M, et al. Phenotypic profile of Ile68Leu transthyretin amyloidosis: an underdiagnosed cause of heart failure. Eur J Heart Fail 2018;20:1417-25.
20. Quarta C, Longhi S, Cappelli F, et al. Late onset cardiomyopathy due to transthyretin Ile68Leu mutation: a cardiogenic variant of familial amyloidosis potentially mimicking sarcomeric hypertrophic cardiomyopathy. European Heart J 2013;34:P2958-P2958.
21. Waddington-Cruz M, Wixner J, Amass L, Kiszko J, Chapman D, Ando Y. THAOS investigators. Characteristics of patients with late-vs early-onset val30met transthyretin amyloidosis from the transthyretin amyloidosis outcomes survey (THAOS). Neurol Ther 2021;10:753-66.
22. Gentile L, Tournev I, Amass L, Chapman D, Mazzeo A. THAOS investigators. phenotypic differences of glu89gln genotype in ATTR amyloidosis from endemic loci: update from THAOS. Cardiol Ther 2021;10:481-90.
23. Parman Y, Adams D, Obici L, et al. European Network for TTR-FAP (ATTReuNET). Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe: where are we now? Curr Opin Neurol 2016;29 Suppl 1:S3-S13.
24. Rapezzi C, Perugini E, Salvi F, et al. Phenotypic and genotypic heterogeneity in transthyretin-related cardiac amyloidosis: towards tailoring of therapeutic strategies? Amyloid 2006;13:143-53.
25. Yamamoto S, Wilczek HE, Nowak G, et al. Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant 2007;7:2597-604.
26. Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med 2015;372:21-9.
27. Sattianayagam PT, Hahn AF, Whelan CJ, et al. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur Heart J 2012;33:1120-7.
28. Mazzeo A, Russo M, Di Bella G, et al. Transthyretin-related familial amyloid polyneuropathy (ttr-fap): a single-center experience in sicily, an italian endemic area. J Neuromuscul Dis 2015;2:S39-48.
29. Mariani LL, Lozeron P, Théaudin M, et al. French Familial Amyloid Polyneuropathies Network (CORNAMYL) Study Group. Genotype-phenotype correlation and course of transthyretin familial amyloid polyneuropathies in France. Ann Neurol 2015;78:901-16.
30. Ihse E, Ybo A, Suhr O, Lindqvist P, Backman C, Westermark P. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol 2008;216:253-61.
31. Gustafsson S, Ihse E, Henein MY, Westermark P, Lindqvist P, Suhr OB. Amyloid fibril composition as a predictor of development of cardiomyopathy after liver transplantation for hereditary transthyretin amyloidosis. Transplantation 2012;93:1017-23.
32. Pilebro B, Suhr OB, Näslund U, Westermark P, Lindqvist P, Sundström T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci 2016;121:17-24.
33. Caponetti AG, Rapezzi C, Gagliardi C, et al. THAOS Investigators. Sex-related risk of cardiac involvement in hereditary transthyretin amyloidosis: insights from THAOS. JACC Heart Fail 2021;9:736-46.
34. Batra J, Rosenblum H, Defilippis EM, et al. Sex differences in the phenotype of transthyretin cardiac amyloidosis due to Val122Ile mutation: insights from noninvasive pressure-volume analysis. J Card Fail 2021;27:67-74.
35. Rapezzi C, Riva L, Quarta CC, et al. Gender-related risk of myocardial involvement in systemic amyloidosis. Amyloid 2008;15:40-8.
36. Hellman U, Alarcon F, Lundgren HE, Suhr OB, Bonaiti-Pellié C, Planté-Bordeneuve V. Heterogeneity of penetrance in familial amyloid polyneuropathy, ATTR Val30Met, in the Swedish population. Amyloid 2008;15:181-6.
37. Iorio A, De Lillo A, De Angelis F, et al. Non-coding variants contribute to the clinical heterogeneity of TTR amyloidosis. Eur J Hum Genet 2017;25:1055-60.
38. Alves-Ferreira M, Azevedo A, Coelho T, et al. Beyond Val30Met transthyretin (TTR): variants associated with age-at-onset in hereditary ATTRv amyloidosis. Amyloid 2021;28:100-6.
39. Polimanti R, Di Girolamo M, Manfellotto D, Fuciarelli M. Functional variation of the transthyretin gene among human populations and its correlation with amyloidosis phenotypes. Amyloid 2013;20:256-62.
40. Musunuru K, Hershberger RE, Day SM, et al. American Heart Association Council on Genomic and Precision Medicine; Council on Arteriosclerosis. Genetic testing for inherited cardiovascular diseases: a scientific statement from the American heart association. Circ Genom Precis Med 2020;13:e000067.
41. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail 2019;7:709-16.
42. Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005;46:1076-84.
43. Musumeci MB, Cappelli F, Russo D, et al. Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis. JACC Cardiovasc Imaging 2020;13:1314-21.
44. Maurer MS, Bokhari S, Damy T, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail 2019;12:e006075.
45. Benson MD, Dasgupta NR, Rao R. Diagnosis and screening of patients with hereditary transthyretin amyloidosis (hATTR): current strategies and guidelines. Ther Clin Risk Manag 2020;16:749-58.
46. Gopal DM, Ruberg FL, Siddiqi OK. Impact of genetic testing in transthyretin (ATTR) cardiac amyloidosis. Curr Heart Fail Rep 2019;16:180-8.
47. Adams D, Ando Y, Beirão JM, et al. Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J Neurol 2021;268:2109-22.
48. Schmidt HH, Barroso F, González-Duarte A, et al. Management of asymptomatic gene carriers of transthyretin familial amyloid polyneuropathy. Muscle Nerve 2016;54:353-60.
49. Obici L, Kuks JB, Buades J, et al. European Network for TTR-FAP (ATTReuNET). Recommendations for presymptomatic genetic testing and management of individuals at risk for hereditary transthyretin amyloidosis. Curr Opin Neurol 2016;29 Suppl 1:S27-35.
50. Guimarães L, Sequeiros J, Skirton H, Paneque M. What counts as effective genetic counselling for presymptomatic testing in late-onset disorders? J Genet Couns 2013;22:437-47.
51. Olsson M, Jonasson J, Cederquist K, Suhr OB. Frequency of the transthyretin Val30Met mutation in the northern Swedish population. Amyloid 2014;21:18-20.
52. Magliano L, Obici L, Sforzini C, et al. ATTRv Collaborators. Psychosocial burden and professional and social support in patients with hereditary transthyretin amyloidosis (ATTRv) and their relatives in Italy. Orphanet J Rare Dis 2021;16:163.
53. Graceffa A, Russo M, Vita GL, et al. Psychosocial impact of presymptomatic genetic testing for transthyretin amyloidotic polyneuropathy. Neuromuscul Disord 2009;19:44-8.
54. Grandis M, Obici L, Luigetti M, et al. Recommendations for pre-symptomatic genetic testing for hereditary transthyretin amyloidosis in the era of effective therapy: a multicenter Italian consensus. Orphanet J Rare Dis 2020;15:348.
55. Conceição I, Damy T, Romero M, et al. Early diagnosis of ATTR amyloidosis through targeted follow-up of identified carriers of TTR gene mutations. Amyloid 2019;26:3-9.
56. Longhi S, Guidalotti PL, Quarta CC, et al. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. JACC Cardiovasc Imaging 2014;7:531-2.
57. Haq M, Pawar S, Berk JL, Miller EJ, Ruberg FL. Can 99mTc-pyrophosphate aid in early detection of cardiac involvement in asymptomatic variant TTR amyloidosis? JACC Cardiovasc Imaging 2017;10:713-4.
58. Leonardi L, Adam C, Beaudonnet G, et al. Skin amyloid deposits and nerve fiber loss as markers of neuropathy onset and progression in hereditary transthyretin amyloidosis. Eur J Neurol 2022;29:1477-87.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Scirpa R, Russo D, Tini G, Sclafani M, Tropea A, Cava F, Autore C, Musumeci B. Clinical translation of genetic testing in TTR Amyloidosis: genotype-phenotype correlations, management of asymptomatic carriers and familial screening. Vessel Plus 2022;6:52. http://dx.doi.org/10.20517/2574-1209.2021.74
AMA Style
Scirpa R, Russo D, Tini G, Sclafani M, Tropea A, Cava F, Autore C, Musumeci B. Clinical translation of genetic testing in TTR Amyloidosis: genotype-phenotype correlations, management of asymptomatic carriers and familial screening. Vessel Plus. 2022; 6: 52. http://dx.doi.org/10.20517/2574-1209.2021.74
Chicago/Turabian Style
Scirpa, Riccardo, Domitilla Russo, Giacomo Tini, Matteo Sclafani, Alessandro Tropea, Francesco Cava, Camillo Autore, Beatrice Musumeci. 2022. "Clinical translation of genetic testing in TTR Amyloidosis: genotype-phenotype correlations, management of asymptomatic carriers and familial screening" Vessel Plus. 6: 52. http://dx.doi.org/10.20517/2574-1209.2021.74
ACS Style
Scirpa, R.; Russo D.; Tini G.; Sclafani M.; Tropea A.; Cava F.; Autore C.; Musumeci B. Clinical translation of genetic testing in TTR Amyloidosis: genotype-phenotype correlations, management of asymptomatic carriers and familial screening. Vessel Plus. 2022, 6, 52. http://dx.doi.org/10.20517/2574-1209.2021.74
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 13 clicks
Cite This Article 13 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.