New endovascular techniques for false lumen thrombosis in aortic dissection after thoracic endovascular aortic repair
Abstract
Over the past decades, both open surgery and endovascular treatment of aortic dissection have made great progress with good clinical outcomes. However, despite this progress, the presence of distal re-entry tears can sustain perfusion of false lumen (FL) and induce aneurysm formation or growth. In particular, about 20% of patients undergoing thoracic endovascular aortic repair (TEVAR) for aortic dissection require reintervention because of incomplete FL thrombosis promoting aortic wall degeneration, post-dissection aortic aneurysm, and rupture. Endovascular techniques to promote FL thrombosis after TEVAR show good early results together with minimal invasiveness, offering different alternatives depending on the case and the level of urgency. Endovascular techniques include FL embolization (with coils, vascular plugs, etc.), candy-plug techniques, parallel stent graft, and branched and fenestrated aortic endoprosthesis. Each of these solutions has advantages and disadvantages. We herein describe the available endovascular options.
Keywords
INTRODUCTION
In 2008, the IRAD group endorsed thoracic endovascular aortic repair (TEVAR) as the gold standard for the treatment of complicated acute type B aortic dissection (TBAD)[1]. Subsequently, several studies clearly confirmed the superiority of TEVAR to surgical or medical therapy in the treatment of this life-threatening circumstance[2,3]. Moreover, during the last decade, the INSTEAD-XL[4] and ABSORB[5] trials demonstrated how TEVAR can play an emergent role in the management of uncomplicated acute TBAD in selected patients with high-risk features, even if many aspects remain to be clarified. Less encouraging results emerged regarding TEVAR for chronic post-dissection thoracoabdominal aortic aneurysms, with complete thrombosis of false lumen (FL) in only 30% of cases[6] and a reintervention rate of 60%[7]. In all these situations, the goal of TEVAR is to cover the intimal tears promoting thrombosis of the FL. However, about 20% of patients undergoing TEVAR of aortic dissection require reintervention because of incomplete FL thrombosis promoting aortic wall degeneration, post-dissection aortic aneurysm, and rupture[8]. Incomplete thrombosis of the FL can affect the long-term survival of these patients, increasing late mortality[9-11]. Over the past decades, endovascular strategies to promote FL thrombosis after TEVAR showed good early results with minimal invasiveness, offering different alternatives according to the anatomy and the level of urgency. The most important endovascular techniques described include FL embolization (with coils, vascular plugs, etc.), candy-plug techniques, branched and fenestrated aortic endoprosthesis, and parallel stent graft technique. Each of these solutions has advantages and disadvantages. In this focused paper, we provide a brief description of endovascular options available to induce false lumen thrombosis after TEVAR in TBAD together with a commentary based on our experience.
FL EMBOLIZATION
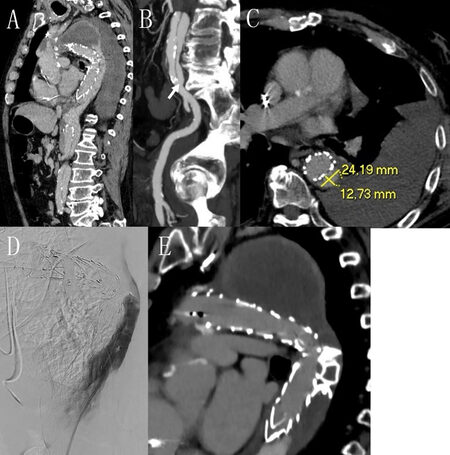
Currently, there are no devices specifically designed for FL embolization. In 2018, Yuan et al. described the FLIRT (false lumen intervention to promote remodeling and thrombosis) concept in aortic dissection, using a combination of patent foramen ovale or atrial septal defect occluders, glue, and coils[12]. In 4/5 cases of persistent flow in FL in patients with TBAD previously treated with TEVAR, complete thrombosis was obtained with the FLIRT concept. Miletic et al. recently described the use of a combination of different devices in FL embolization, including iliac plugs, coils, and nitinol plugs in 51 patients, with a favorable aortic remodeling (FL thrombosis with ≥ 10% decrease in diameter and ≥ 10% increase in true lumen diameter) achieved in 39.2% of cases[13]. Moreover, a complete obliteration of the entire FL was obtained in six patients, with only nine patients requiring a second procedure for incomplete thrombosis of FL and aortic diameter increase. In all these and other studies, FL embolization proved to be feasible and safe. Technically, the most important aspect of the intervention is a meticulous study of the pre-procedural CT scan, in order to plan how to “earn” the FL and choose the right devices, based on the anatomy of the dissection [Figure 1]. Moreover, to choose the size, type, and number of all devices available for the embolization of the false lumen, fundamental is the measurement of the diameter of the false lumen above the celiac trunk and its longitudinal extension and the angiographic check during the procedure to ensure the effectiveness of the embolization. Usually, during this procedure, we gain the false lumen using first a 0.035 floppy guide wire with a diagnostic peripheral catheter (e.g., cobra, vertebral, multipurpose, or Simmons, depending on the anatomy of the dissection). Then, once in the false lumen, it can be useful to place an introducer sheath to give more stability to the system as well as the possibility to use a diagnostic catheter or a microcatheter for the deployment of coils and embolic liquid, or directly the introducer sheath for the deployment of other devices such as vascular plugs. If suitable, a combination of all devices should be considered to stop the backflow in the false lumen.
Figure 1. Multiplanar reconstruction of CT scan showing retrograde perfusion of the false lumen with a large post-dissection thoracic aneurysm and haemothorax (A); identification of intimal tear to earn the false lumen (B) (arrow) and correct measurement of the false lumen diameter (C) are mandatory to plan the procedure; and intraprocedural angiography (D) and CT scan control at discharge (E) showing complete exclusion and thrombosis of the false lumen with the use of two vascular plugs.
The prompt availability of the devices and their low costs make the embolization of the false lumen advantageous, especially in the management of selected urgent and emergency cases.
CANDY-PLUG TECHNIQUE
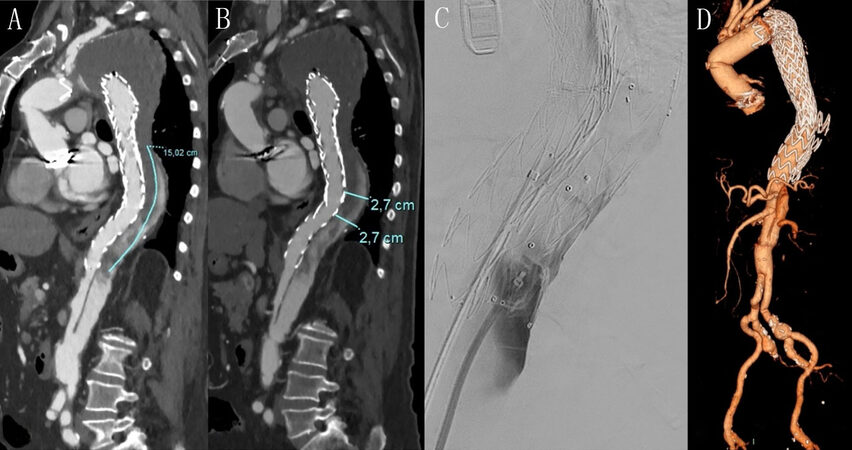
In 2013, Kölbel et al. first described the candy-plug technique for FL occlusion, using a combination of Zenith TX2® endovascular graft (Cook Medical, Bjæverskov, Denmark) and nitinol vascular plugs[14]. Since then, different approaches have been used to reproduce the candy-plug technique, using the second generation of candy-plugs (without the necessity to complete the procedure with a vascular plug) or different homemade candy-plug devices[15,16]. Eleshra et al. recently described the use of the second-generation candy-plug in the treatment of 14 chronic aortic dissection, with a favorable aortic remodeling achieved in eight patients and only two patients requiring reintervention[17]. The rationale of this technique is the placement of the candy-plug into the false lumen, parallel to the stent graft present in the true lumen, immediately above the celiac trunk. For the FL embolization, a careful evaluation of the preoperative CT scan is essential to understand the feasibility of the intervention (available access to the false lumen and its sufficient extension above the celiac trunk) and the correct oversizing of the candy-plug. For this purpose, the measurement of the length of the dissection and the largest diameter of the FL above the celiac trunk is mandatory [Figure 2]. Since these are custom-made devices, obviously this technique is preferred in non-urgent conditions and if the anatomy allows it. In particular, we believe that the maximum diameter of the false lumen above the celiac trunk must not exceed 36-38 mm and in this tract, the aorta must not be tortuous. Oversizing of 15%-25% of the false lumen diameter above the celiac trunk should be sufficient to obtain a good sealing of the device[18], reducing the risk of injuries to the aortic wall.
BRANCHED AND FENESTRATED AORTIC ENDOPROSTHESIS
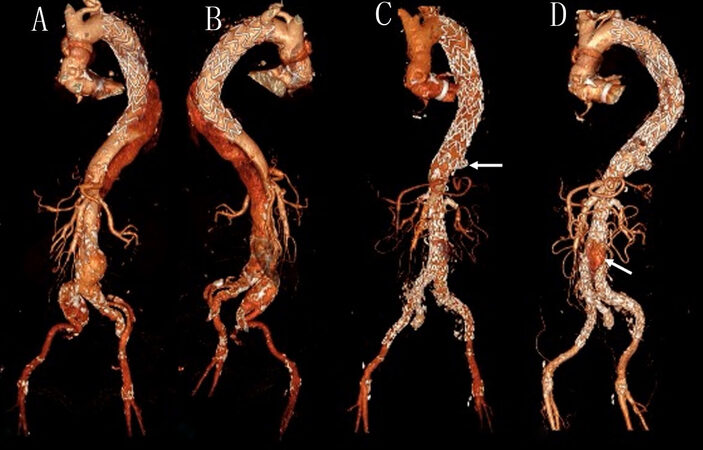
Branched and fenestrated stent grafts (BEVAR and FEVAR techniques) have increasingly become safe and effective options for the treatment of thoracoabdominal aortic aneurysm (TAAA) and in selected cases with specific anatomical conditions are considered the first-line treatment option[19]. Unfortunately, their use to promote thrombosis of the false lumen in TBAD previously treated with TEVAR has severe anatomical limitations, primarily because the stiff and thickened intimal flap can limit the success of this strategy[20]. Furthermore, the true lumen may be very narrow, and the visceral vessels can arise from the FL or true lumen, making BEVAR/FEVAR technically very demanding[21] [Figure 3]. Moreover, BEVAR and FEVAR, compared to the other described endovascular techniques, have a higher risk of complications, such as injuries or ischemia of the parenchyma due to the manipulation of the visceral vessels and the patency of the visceral stent grafts. Technically, fundamental is the right choice of the main graft and the bridging stent, based on the anatomy of the aorta and visceral vessels, such as their diameter, extension, peripheral ramifications, and relationship to the true and false lumens. In the planning of this type of procedure, it may be useful, based on our experience, to consider the design of hybrid solutions (BEVAR and FEVAR) or the use of inner branch and retro branch [Figure 4], to reduce the encumbrance within an often small true lumen. Moreover, the use of preloaded guided wires could reduce the difficulty of the operation. In the case of using branched solutions, it is preferable to use self-expanding visceral stents, with greater conformability; in the case of FEVAR, the use of balloon expandable stents should be preferred to reduce the risk of type IIIc endoleak.
Figure 3. CT volume rendering reconstruction of post-dissection thoraco-abdominal aneurysm with a very narrow true lumen and visceral arteries all originated from the true lumen (A, B); patient was treated with BEVAR and positioning of coils in the false lumen in the diaphragmatic tract (arrow) (C); and false lumen was thrombosed in the thoracic and suprarenal abdominal aorta, and a small type II endoleak was detected in the subrenal aorta (arrow) (D).
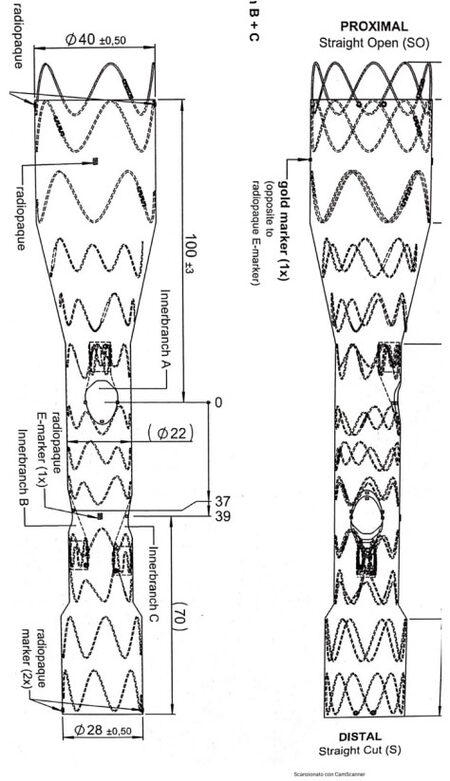
Figure 4. Schematic rappresentation of a branched endoprosthetis designed with retrobranch for renal arteries (Innerbranch B and Innerbranch C) and anterograde inner branch for mesenteric superior artery (Innerbranch A). The diameter of the proximal extremity (40 mm), the middle tract (2 mm), and the distal extremity (28 mm) of the endoprosthesis are indicated. Moreover, the distance between the proximal neck and Innerbranch A is shown (100 mm), as well as the distance between Innerbranch A and Innerbranch B (37 mm), between Innerbranch A and Innerbranch C (39 mm), and between Innerbranch C and the distal neck of the device (70 mm). The radiopaque marker along the main body of the endoprosthesis and the inner branch are also indicated to ensure the correct orientation of the device during the deployment.
Despite these limitations, through the continuous development of this emergent technique, in the future, BEVAR/FEVAR will increasingly be used for the treatment of post-dissection thoracoabdominal aortic aneurysms.
PARALLEL STENT GRAFT TECHNIQUE
The parallel stent graft technique (snorkel/chimney) [Figure 5] represents a valid off-the-shelf alternative in the treatment of complex abdominal and thoracic aortic pathologies[22,23]. Liu et al. recently described a series of 21 patients with chronic post-dissection thoracoabdominal aortic aneurysms treated with this technique[24]. Technical success was achieved in 17/21 (four intraoperative type I endoleaks spontaneously resolved within one month), complete thrombosis of the FL was obtained in 19/21 with a patency rate of the visceral stent graft at 18 months of 100% for the arteries anterogradely revascularized and 91.2% for the retrogradely revascularized. The advantages of this technique reside in the ready availability of all the necessary “armamentarium”, with the possibility of treating emergency situations with different and multiple solutions according to the anatomy of the aorta and visceral vessels. The disadvantages are the demanding procedure, in particular in the choice of the correct oversizing of the aortic stent graft as well as of the type of visceral stent graft (balloon versus self-expandable and exact length and diameter) and, as for BEVAR/FEVAR solutions, the narrowness and stiffness of the true lumen. If the true lumen is too narrow to receive more than two stent grafts, an anterograde approach is recommended (preferably the left brachial or axillary artery) for the reconstruction of the mesenteric superior artery and celiac trunk, as well as a retrograde access for the renal arteries. Intentional coverage of celiac trunk and small accessories renal arteries (< 5 mm) should be considered, especially in emergency cases. Moreover, a self-expanding bare metal stent can be added to reduce the risk of compression or kinking of the parallel stent graft. However, long-term outcomes (especially patency of visceral branches) and large sample size studies are needed to evaluate the safety and efficacy of this technique in the treatment of post-dissection thoracoabdominal aortic aneurysms.
Figure 5. Drawing illustrating the parallel stent graft technique to stop retrograde flow in the thoracic false lumen from residual intimal tears in the suprarenal and subrenal aorta (black arrows). Three stent grafts are positioned from the origin of the left subclavian artery to the aortic bifurcation. The visceral stent of the celiac trunk and the superior mesenteric artery are positioned between the first and second aortic stent grafts; instead, the stents of renal arteries are located between the second and third aortic stent grafts. All visceral are incannulated and stented with the anterograde approach from the top.
COMMENTARY AND CONCLUSION
Incomplete thrombosis of the FL with persistent backflow from distal re-entry tears is one of the major limitations of TEVAR in TBAD[25] and is independently associated with poor long-term survival. Over the last few years, different techniques such as STABILIZE and Knickerbocker[26,27] have been developed to prevent this fearful complication already from the first treatment, especially in the acute phase when, due to the elasticity and minor thickness of the intimal flap, it is possible to destroy it, in order to create a single lumen and stop backflow in the FL. In this article, we do not voluntarily describe these techniques, because, in our opinion, they are most effective if applied in the acute/subacute phase, in relation to the greater elasticity of the intimal flap, as already reported. We therefore focus this review on the endovascular techniques currently used to induce direct thrombosis of the FL lumen in aortic dissections previously treated with TEVAR. In our experience, the factors that most affect the choice of one specific technique are the degree of emergency and the anatomy of the dissection (extent and size of the true and false lumen and origin of the visceral vessels). According to our practice, in emergency conditions and when anatomy allows it, direct embolization techniques with plug and coil or still other off-the-shelf solutions (e.g., the parallel stent graft technique or homemade candy-plug) are preferred, considering their ready availability as well as costs. In non-emergency conditions, customized solutions such as candy-plug and BEVAR/FEVAR could represent a valid “ad hoc” solution for each patient, along with the possibility of using a combination of the above-mentioned techniques. In conclusion, endovascular strategies to promote FL thrombosis after TEVAR in aortic dissection showed good early results with minimal invasiveness, offering different alternative depending on the anatomy and the level of urgency. In any case, in consideration of the complexity of the problem, each of these strategies should be used with caution and more long-term studies and prospective and randomized clinical trials are needed to provide solid evidence on this topic.
DECLARATIONS
Authors’ contributions
Make substantial contributions to conception and design of the study and perform data analysis and interpretation: Buia F, Russo V, Lovato L
Perform data acquisition, as well as provide administrative, technical, and material support: Attinà D, Niro F
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
REFERENCES
1. Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option? JACC Cardiovasc Interv 2008;1:395-402.
2. Zeeshan A, Woo EY, Bavaria JE, et al. Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional open surgical and medical therapy. J Thorac Cardiovasc Surg 2010;140:S109-15; discussion S142.
3. Jonker FH, Patel HJ, Upchurch GR, et al. Acute type B aortic dissection complicated by visceral ischemia. J Thorac Cardiovasc Surg 2015;149:1081-6.e1.
4. Nienaber CA. Influence and critique of the INSTEAD Trial (TEVAR versus medical treatment for uncomplicated type B aortic dissection). Semin Vasc Surg 2011;24:167-71.
5. Brunkwall J, Kasprzak P, Verhoeven E, et al. ADSORB Trialists. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg 2014;48:285-91.
6. Mani K, Clough RE, Lyons OT, et al. Predictors of outcome after endovascular repair for chronic type B dissection. Eur J Vasc Endovasc Surg 2012;43:386-91.
7. Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632-47.
8. Zhang S, Chen Y, Zhang Y, et al. Should the distal tears of aortic dissection be treated? Int J Cardiol 2018;261:162-6.
9. Song SW, Kim TH, Lim SH, Lee KH, Yoo KJ, Cho BK. Prognostic factors for aorta remodeling after thoracic endovascular aortic repair of complicated chronic DeBakey IIIb aneurysms. J Thorac Cardiovasc Surg 2014;148:925-32, 933.e1; discussion 932.
10. Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aorticdissection with patent false lumen: predictive role of entrytear size and location. Circulation 2012; 125:3133-41.
11. Akutsu K, Nejima J, Kiuchi K, et al. Effects of the patent false lumen on the long-term outcome of type B acute aortic dissection. Eur J Cardiothorac Surg 2004;26:359-66.
12. Yuan X, Mitsis A, Semple T, et al. False lumen intervention to promote remodelling and thrombosis-The FLIRT concept in aortic dissection. Catheter Cardiovasc Interv 2018;92:732-40.
13. Miletic KG, Kindzelski BA, Hodges KE, et al. Impact of endovascular false lumen embolization on thoracic aortic remodeling in chronic dissection. Ann Thorac Surg 2021;111:495-501.
14. Kölbel T, Lohrenz C, Kieback A, Diener H, Debus ES, Larena-Avellaneda A. Distal false lumen occlusion in aortic dissection with a homemade extra-large vascular plug: the candy-plug technique. J Endovasc Ther 2013;20:484-9.
15. Rohlffs F, Spanos K, Tsilimparis N, Debus ES, Kölbel T. Techniques and outcomes of false lumen embolization in chronic type B aortic dissection. J Cardiovasc Surg (Torino) 2018;59:784-8.
16. Ogawa Y, Nishimaki H, Chiba K, et al. Clinical utility of the candy-plug technique using an excluder aortic extender. Ann Vasc Dis 2021;14:139-45.
17. Eleshra A, Kölbel T, Tsilimparis N, et al. Candy-plug generation II for false lumen occlusion in chronic aortic dissection: feasibility and early results. J Endovasc Ther 2019;26:782-6.
18. Rohlffs F, Tsilimparis N, Fiorucci B, et al. The candy-plug technique: technical aspects and early results of a new endovascular method for falselumen occlusion in chronic aortic dissection. J Endovasc Ther 2017;24:549-555.
19. Verhoeven EL, Katsargyris A, Bekkema F, et al. Editor’s choice - ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: results from 166 consecutive patients. Eur J Vasc Endovasc Surg 2015;49:524-31.
20. Zeng Z, Zhao Y, Wu M, et al. Endovascular strategies for post-dissection aortic aneurysm (PDAA). J Cardiothorac Surg 2020;15:287.
22. Donas KP, Lee JT, Lachat M, Torsello G, Veith FJ. PERICLES investigators. Collected world experience about the performance of the snorkel/chimney endovascular technique in the treatment of complex aortic pathologies: the PERICLES registry. Ann Surg 2015;262:546-53; discussion 552.
23. Moulakakis KG, Mylonas SN, Dalainas I, et al. The chimney-graft technique for preserving supra-aortic branches: a review. Ann Cardiothorac Surg 2013;2:339-46.
24. Liu J, Li Z, Feng J, et al. Total endovascular repair with parallel stent-grafts for postdissection thoracoabdominal aneurysm after prior proximal repair. J Endovasc Ther 2019;26:668-75.
25. Li D, Ye L, He Y, et al. False lumen status in patients with acute aortic dissection: a systematic review and meta-analysis. J Am Heart Assoc 2016;5:e003172.
26. Faure EM, El Batti S, Sutter W, et al. Stent-assisted balloon-induced intimal disruption and relamination of distal remaining aortic dissection after acute DeBakey type I repair. J Thorac Cardiovasc Surg 2019;157:2159-65.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Buia F, Russo V, Attinà D, Niro F, Lovato L. New endovascular techniques for false lumen thrombosis in aortic dissection after thoracic endovascular aortic repair. Vessel Plus 2022;6:48. http://dx.doi.org/10.20517/2574-1209.2022.04
AMA Style
Buia F, Russo V, Attinà D, Niro F, Lovato L. New endovascular techniques for false lumen thrombosis in aortic dissection after thoracic endovascular aortic repair. Vessel Plus. 2022; 6: 48. http://dx.doi.org/10.20517/2574-1209.2022.04
Chicago/Turabian Style
Buia, Francesco, Vincenzo Russo, Domenico Attinà, Fabio Niro, Luigi Lovato. 2022. "New endovascular techniques for false lumen thrombosis in aortic dissection after thoracic endovascular aortic repair" Vessel Plus. 6: 48. http://dx.doi.org/10.20517/2574-1209.2022.04
ACS Style
Buia, F.; Russo V.; Attinà D.; Niro F.; Lovato L. New endovascular techniques for false lumen thrombosis in aortic dissection after thoracic endovascular aortic repair. Vessel Plus. 2022, 6, 48. http://dx.doi.org/10.20517/2574-1209.2022.04
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 6 clicks
Cite This Article 6 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.