Predictors and risk-adjusted outcomes of new-onset postoperative atrial fibrillation in repeat surgical and valve-in-valve transcatheter aortic valve replacement
Abstract
Aim: New-onset postoperative atrial fibrillation/flutter (POAF/AFL) complications have not been well studied for repeat aortic valve replacements (r-AVR); this study identified risk factors predisposing to POAF/AFL and POAF/AFL’s effect upon risk-adjusted outcomes.
Methods: Using New York State’s Statewide Planning and Research Cooperative System records (2005-2018), multivariable forward selection models identified risks predictive of POAF/AFL. To identify POAF/AFL’s impact upon risk-adjusted mortality/morbidity (MM) and 30-day readmission (READMIT), forward selection logistic regression models applied Firth bias correction to address data sparsity.
Results: Of the 242 r-AVR patients, 147 underwent repeat surgical aortic valve replacements (r-SAVR) and 95 underwent valve-in-valve transcatheter aortic valve replacements (ViV-TAVR); 39.46% of r-SAVR and 43.16% of ViV-TAVR patients had POAF/AFL. R-SAVR patients with POAF/AFL were older (69.7 ± 11.1 vs. 56.7 ± 13.2 years,
Bivariately, POAF/AFL was associated with READMIT, but not MM. Correspondingly, multivariable models found POAF/AFL increased READMIT (OR: 3.12, 95%CI: 1.46-6.65, P < 0.01), but not MM. However, black race (OR: 4.97,
Conclusion: More common in older and cerebrovascular disease patients, 41% of r-AVR patients with POAF/AFL had increased READMIT risk; thus, future investigations should focus on improving POAF/AF r-AVR patients’ post-discharge continuity of care.
Keywords
INTRODUCTION
Across all cardiothoracic surgical procedures, new-onset postoperative atrial fibrillation or atrial flutter (POAF/AFL) occurs commonly. In 2019, surgical aortic valve replacements (SAVR) and transcatheter aortic valve replacement (TAVR) were the most common treatments for aortic stenosis, with over 130,000 patients who underwent an initial AVR procedure; since 2011, these aortic valve replacement (AVR) volumes have dramatically increased[1-5]. New-onset POAF/AFL is not always a transient condition; even for patients discharged in normal sinus rhythm, recurrent atrial fibrillation (AF) has been reported up to 5 years
As aortic valves inherently have limited durability, bioprosthetic valves often experience structural deterioration within 10-12 years, and thus require repeat procedures[7]. For repeat AVR (r-AVR) patients, the POAF/AFL incidence was reportedly increased for more invasive procedures (35.5%-60% of SAVR and 10.4%-50.4% of TAVR patients); POAF/AFL has been associated with greater mortality, stroke, and hospital resource utilization[6,8-18]. For example, one single-center study has shown 63.6% of 22 r-SAVR and 18.2% of 22 valve-in-valve transcatheter aortic valve replacements (ViV-TAVR) patients to have POAF/AFL[19]. In spite of these increased r-AVR procedural rates, r-AVR patients’ risk factors associated with POAF/AFL, as well as the impact of new-onset POAF/AFL upon short-term r-AVR patients’ outcomes, have not been previously reported. As a novel investigation, therefore, this study was specifically designed to address this knowledge gap.
Using the New York State’s Statewide Planning and Research Cooperative System (SPARCS) database records from 2005-2018, this observational, retrospective cohort analysis identified the patient risk factors predictive of r-AVR POAF/AFL, as well as the POAF/AFL impact upon risk-adjusted 30-day morbidity/mortality (MM) composite and 30-day readmission (READMIT). After holding other patient risk factors and procedural details constant, the study’s hypothesis was that POAF/AFL may be an important post-procedural complication contributing to increased risk of MM and READMIT.
METHODS
Study population
Within New York, the 2018 population of adults was estimated to be approximately 19 million[20]. Since 2003, the New York State’s SPARCS database has tracked all non-federal hospital-based inpatient and outpatient care, ambulatory surgery, and emergency room care; patients’ records include their demographic information, diagnoses, procedures, and outcomes[21]. Using billing codes [Supplementary Tables 1 and 2,] the New York State’s SPARCS records for New York State residents undergoing repeat aortic valve (r-AVR) procedures from January 2005 to November 2018 were extracted. Given that only de-identified SPARCS reports were received by the study team, the Stony Brook University Committee on Research in Human Subjects provided a “not human study research” written exemption [IRBNet #: IRB2021-00605 - POAF
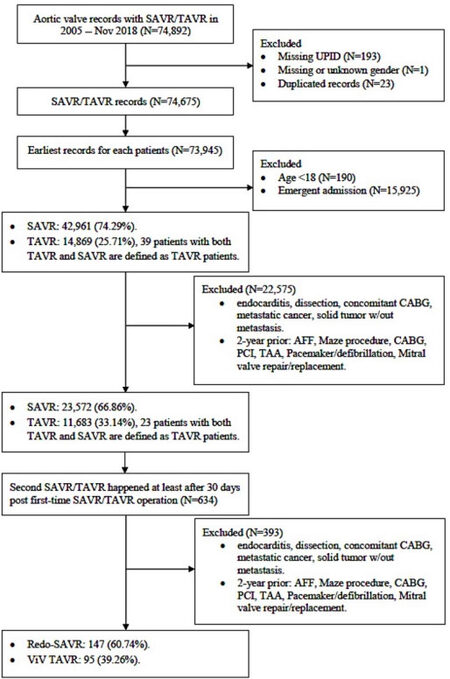
As this investigation’s procedure of interest, repeat aortic valve procedures were defined as a second SAVR or TAVR that occurred at least 30 days after their first SAVR or TAVR operation. Based on patients’ r-AVR procedure date, all encounters occurring within 30-day of discharge were recorded; 30-day readmissions and repeat procedures were identified. Duplicate records and patients without unique personal identifiers or gender information were excluded. Given the inherently higher risk of complications, patients with endocarditis, thoracic aortic aneurysms with or without dissection, coronary artery bypass grafting, percutaneous coronary intervention, mitral valve repair or replacement, metastatic cancer, any solid tumor without metastasis, or r-AVR procedures with concomitant coronary artery bypass grafting procedure were excluded. To identify patients with new-onset POAF/AFL, patients with a history of atrial fibrillation or flutter, Maze procedure, pacemaker implantation or defibrillation two years prior to their first AVR operation and r-AVR operation were excluded. The flow diagram of our patient population selection is shown in [Figure 1]. After identifying all r-AVR patients who met the inclusion and exclusion criteria,
Figure 1. Data extraction flowchart. SAVR: Surgical aortic valve replacements; TAVR: transcatheter aortic valve replacements.
Descriptive table of r-SAVR and ViV TAVR patients’ baseline characteristics, risk factors, and Elixhauser comorbidities by surgery type
| Variable | Total | r-SAVR | ViV-TAVR | P-value* |
| Patients’ characteristics | ||||
| Type of admission Elective Urgent | 78.93% 21.07% | 82.99% 17.01% | 72.63% 27.37% | 0.05 |
| Gender Female Male | 39.67% 60.33% | 36.05% 63.95% | 45.26% 54.74% | 0.15 |
| Age (years) | 66.60 ± 14.32 | 61.82 ± 13.91 | 74.00 ± 11.57 | < 0.01 |
| Race Black Other | 6.20% 93.80% | 7.48% 92.52% | 4.21% 95.79% | 0.42 |
| Ethnicity Hispanic Other/unknown | 4.55% 95.45% | 5.44% 94.56% | 3.16% 96.84% | 0.53 |
| Insurance Commercial Medicaid/Other Medicare | 36.36% 2.89% 60.74% | 45.58% 4.08% 50.34% | 22.11% 1.05% 76.84% | < 0.01 |
| Risk factors | ||||
| Post-op atrial fibrillation/flutter | 40.91% | 39.46% | 43.16% | 0.57 |
| Tobacco/Smoking | 41.74% | 38.10% | 47.37% | 0.15 |
| Hypertension | 78.10% | 73.47% | 85.26% | 0.03 |
| Congestive heart failure | 30.58% | 18.37% | 49.47% | < 0.01 |
| Cardiomyopathy | 9.09% | 6.12% | 13.68% | 0.05 |
| Diabetes mellitus | 3.72% | 4.08% | 3.16% | 0.75 |
| Coronary artery disease | 46.28% | 28.57% | 73.68% | < 0.01 |
| Chronic lung disease | 27.69% | 23.81% | 33.68% | 0.09 |
| COPD | 14.46% | 7.48% | 25.26% | < 0.01 |
| History of stroke | 7.44% | 6.80% | 8.42% | 0.64 |
| Stroke | 9.92% | 8.84% | 11.58% | 0.49 |
| Carotid disease | 2.07% | 0.68% | 4.21% | 0.08 |
| Carotid stenosis | 1.65% | 0.68% | 3.16% | 0.30 |
| Cerebral vascular disease | 16.12% | 13.61% | 20.00% | 0.19 |
| Peripheral vascular disease | 5.37% | 2.72% | 9.47% | 0.04 |
| History of MI | 6.20% | 2.72% | 11.58% | 0.04 |
| MI | 11.16% | 6.80% | 17.89% | 0.01 |
| Depression | 9.09% | 7.48% | 11.58% | 0.28 |
| Bipolar disorder | 1.24% | 2.04% | 0.00% | 0.28 |
| Schizophrenia | 0.41% | 0.00% | 1.05% | 0.39 |
| Dementia | 2.07% | 0.00% | 5.26% | 0.01 |
| Bicuspid aortic valve | 0.83% | 1.36% | 0.00% | 0.51 |
| Dyspnea | 3.31% | 2.72% | 4.21% | 0.71 |
| Chest pain | 2.89% | 3.40% | 2.11% | 0.71 |
| Dialysis | 3.31% | 2.04% | 5.26% | 0.26 |
| Hyperlipidemia | 57.02% | 49.66% | 68.42% | < 0.01 |
| Presence of prosthetic heart valve | 11.57% | 12.93% | 9.47% | 0.41 |
| Abdominal aortic aneurysm | 2.07% | 2.72% | 1.05% | 0.66 |
| Non-rheumatic aortic stenosis | 63.64% | 61.90% | 66.32% | 0.49 |
| Rheumatic heart disease | 6.61% | 4.76% | 9.47% | 0.15 |
| Obstructive sleep apnea | 8.26% | 9.52% | 6.32% | 0.40 |
| Lymphoma | 0.83% | 1.36% | 0.00% | 0.52 |
| Chronic kidney disease Stage 3 Stage 4 ESRD | 11.57% 1.24% 5.79% | 6.12% 0.68% 4.76% | 20.00% 2.11% 7.37% | < 0.01 |
| CKD, with dialysis | 3.31% | 2.04% | 5.26% | 0.27 |
| CKD, without dialysis | 23.55% | 14.97% | 36.84% | < 0.01 |
| Obesity | 22.73% | 18.37% | 29.47% | 0.04 |
| Iron deficiency anemia | 13.22% | 12.93% | 13.68% | 0.86 |
| Rheumatoid arthritis/collagen vascular diseases | 4.96% | 5.44% | 4.21% | 0.77 |
| Fluid and electrolyte disorders | 1.65% | 2.04% | 1.05% | 0.65 |
| Pulmonary hypertension | 20.66% | 16.33% | 27.37% | 0.04 |
| Thrombocytopenia | 28.51% | 33.33% | 21.05% | 0.04 |
| Previous internal cardioverter-defibrillator | 3.31% | 0.68% | 7.37% | 0.01 |
| Hypothyroidism | 13.64% | 12.24% | 15.79% | 0.43 |
| Intra-aortic balloon pump | 2.89% | 2.72% | 3.16% | 1.00 |
Descriptive table of r-SAVR and ViV TAVR patients’ baseline characteristics, risk factors, and comorbidities by POAF/AFL
| r-SAVR | ViV-TAVR | |||||||
| Variable | Total | POAF/AFL | No POAF/AFL | P-value* | Total | POAF/AFL | No POAF/AFL | P-value* |
| Patients’ characteristics | ||||||||
| Type of admission Elective Urgent | 82.99% 17.01% | 79.31% 20.69% | 85.39% 14.61% | 0.34 | 72.63% 27.37% | 73.17% 26.83% | 72.22% 27.78% | 0.92 |
| Gender Female Male | 36.05% 63.95% | 44.83% 55.17% | 30.34% 69.66% | 0.07 | 45.26% 54.74% | 51.22% 48.78% | 40.74% 59.26% | 0.31 |
| Age (years) | 61.82 ± 13.91 | 69.71 ± 11.09 | 56.69 ± 13.19 | < 0.01 | 74.00 ± 11.57 | 74.61 ± 9.65 | 73.54 ± 12.91 | 0.64 |
| Race Black Other | 7.48% 92.52% | 12.07% 87.93% | 4.49% 95.51% | 0.11 | 4.21% 95.79% | 4.88% 95.12% | 3.70% 96.30% | 1.00 |
| Ethnicity Hispanic Other/unknown | 5.44% 94.56% | 3.45% 96.55% | 6.74% 93.26% | 0.48 | 3.16% 96.84% | 4.88% 95.12% | 1.85% 98.15% | 0.58 |
| Insurance Commercial Medicaid/Other Medicare | 45.58% 4.08% 50.34% | 29.31% 0.00% 70.69% | 56.18% 6.74% 37.08% | < 0.01 | 22.11% 1.05% 76.84% | 21.95% 0.00% 78.05% | 22.22% 1.85% 75.93% | 1.00 |
| Tobacco/Smoking | 38.10% | 37.93% | 38.20% | 0.97 | 47.37% | 53.66% | 42.59% | 0.28 |
| Hypertension | 73.47% | 75.86% | 71.91% | 0.60 | 85.26% | 82.93% | 87.04% | 0.58 |
| Congestive heart failure | 18.37% | 25.86% | 13.48% | 0.06 | 49.47% | 53.66% | 46.30% | 0.48 |
| Cardiomyopathy | 6.12% | 6.90% | 5.62% | 1.00 | 13.68% | 17.07% | 11.11% | 0.40 |
| Diabetes mellitus | 4.08% | 3.45% | 4.49% | 1.00 | 3.16% | 2.44% | 3.70% | 1.00 |
| Coronary artery disease | 28.57% | 39.66% | 21.35% | 0.02 | 73.68% | 80.49% | 68.52% | 0.19 |
| Chronic lung disease | 23.81% | 18.97% | 26.97% | 0.27 | 3.16% | 2.44% | 3.70% | 1.00 |
| COPD | 7.48% | 6.90% | 7.87% | 1.00 | 8.42% | 12.20% | 5.56% | 0.52 |
| History of stroke | 6.80% | 8.62% | 5.62% | 0.48 | 1.05% | 2.44% | 0.00% | 0.28 |
| Stroke | 8.84% | 10.34% | 7.87% | 0.60 | 11.58% | 19.51% | 5.56% | 0.05 |
| Carotid disease | 0.68% | 0.00% | 1.12% | 1.00 | 4.21% | 7.32% | 1.85% | 0.31 |
| Carotid stenosis | 0.68% | 0.00% | 1.12% | 1.00 | 3.16% | 4.88% | 1.85% | 0.56 |
| Cerebral vascular disease | 13.61% | 17.24% | 11.24% | 0.30 | 20.00% | 31.71% | 11.11% | 0.01 |
| Peripheral vascular disease | 2.72% | 3.45% | 2.25% | 1.00 | 9.47% | 9.76% | 9.26% | 1.00 |
| History of MI | 2.72% | 6.90% | 0.00% | 0.02 | 11.58% | 14.63% | 9.26% | 0.42 |
| MI | 6.80% | 6.90% | 6.74% | 1.00 | 17.89% | 21.95% | 14.81% | 0.37 |
| Depression | 7.48% | 5.17% | 8.99% | 0.52 | 11.58% | 12.20% | 11.11% | 0.87 |
| Bipolar disorder | 2.04% | 3.45% | 1.12% | 0.56 | - | - | - | - |
| Schizophrenia | - | - | - | - | 1.05% | 0.00% | 1.85% | 1.00 |
| Dementia | - | - | - | - | 5.26% | 7.32% | 3.70% | 0.65 |
| Bicuspid aortic valve | 1.36% | 0.00% | 2.25% | 0.52 | - | - | - | - |
| Dyspnea | 2.72% | 3.45% | 2.25% | 1.00 | 4.21% | 4.88% | 3.70% | 1.00 |
| Chest pain | 3.40% | 5.17% | 2.25% | 0.38 | 2.11% | 4.88% | 0.00% | 0.1900 |
| Dialysis | 2.04% | 5.17% | 0.00% | 0.06 | 5.26% | 4.88% | 5.56% | 1.0000 |
| Hyperlipidemia | 49.66% | 58.62% | 43.82% | 0.08 | 68.42% | 70.73% | 66.67% | 0.67 |
| Presence of prosthetic heart valve | 12.93% | 13.79% | 12.36% | 0.80 | 9.47% | 9.76% | 9.26% | 1.00 |
| Abdominal aortic aneurysm | 2.72% | 3.45% | 2.25% | 1.00 | 1.05% | 0.00% | 1.85% | 1.00 |
| Non-rheumatic aortic stenosis | 61.90% | 55.17% | 66.29% | 0.17 | 66.32% | 70.73% | 62.96% | 0.43 |
| Rheumatic heart disease | 4.76% | 8.62% | 2.25% | 0.12 | 9.47% | 9.76% | 9.26% | 1.00 |
| Obstructive sleep apnea | 9.52% | 0.00% | 15.73% | < 0.01 | 6.32% | 2.44% | 9.26% | 0.24 |
| Lymphoma | 1.36% | 1.72% | 1.12% | 1.00 | - | - | - | - |
| Chronic kidney disease Stage 3 Stage 4 ESRD | 6.12% 0.68% 4.76% | 10.34% 1.72% 6.90% | 3.37% 0.00% 3.37% | 0.09 | 20.00% 2.11% 7.37% | 21.95% 0.00% 4.88% | 18.52% 3.70% 9.26% | 0.59 |
| CKD, with dialysis | 2.04% | 5.17% | 0.00% | 0.06 | 5.26% | 4.88% | 5.56% | 1.00 |
| CKD, without dialysis | 14.97% | 22.41% | 10.11% | 0.04 | 36.84% | 39.02% | 35.19% | 0.70 |
| Obesity | 18.37% | 15.52% | 20.22% | 0.47 | 29.47% | 24.39% | 33.33% | 0.34 |
| Iron deficiency anemia | 12.93% | 12.07% | 13.48% | 0.80 | 13.68% | 17.07% | 11.11% | 0.40 |
| Rheumatoid arthritis/collagen vascular diseases | 5.44% | 6.90% | 4.49% | 0.71 | 4.21% | 2.44% | 5.56% | 0.63 |
| Fluid and electrolyte disorders | 2.04% | 5.17% | 0.00% | 0.06 | 1.05% | 2.44% | 0.00% | 0.44 |
| Pulmonary hypertension | 16.33% | 18.97% | 14.61% | 0.48 | 27.37% | 36.59% | 20.37% | 0.08 |
| Thrombocytopenia | 33.33% | 34.48% | 32.58% | 0.81 | 21.05% | 24.39% | 18.52% | 0.49 |
| Previous internal cardioverter-defibrillator | 0.68% | 1.72% | 0.00% | 0.40 | 7.37% | 9.76% | 5.56% | 0.70 |
| Hypothyroidism | 12.24% | 12.07% | 12.36% | 0.96 | 15.79% | 14.63% | 16.67% | 0.79 |
| Intra-aortic balloon pump | 2.72% | 5.17% | 1.12% | 0.30 | 3.16% | 2.44% | 3.70% | 1.00 |
Outcome measures
For this study, the co-primary outcomes included predictors of new-onset postoperative atrial fibrillation and/or atrial flutter (POAF/AFL), outcomes of r-AVR patients with POAF/AFL, and predictors of a combined mortality and morbidity composite endpoint (MM), and an indicator of 30-day hospital readmission (READMIT). Based on the Society of Thoracic Surgeons’ (STS) standardized national Adult Cardiac Surgery Database reports’ short-term outcomes, this study’s primary MM composite endpoint was comprised of 30-day operative mortality (i.e., either a death in-hospital or within 30 days) or any major morbidity (based upon any the following events occurring: new perioperative stroke, new renal failure requiring dialysis, extended post-procedural ventilator use, deep sternal wound infection, and/or repeat cardiac procedure) as shown in [Table 3].
Descriptive table of outcomes after r-AVR by POAF/AFL and surgery type
| r-SAVR | ViV-TAVR | |||||||
| Variable | Total | POAF/AFL | No POAF/AFL | P-value* | Total | POAF/AFL | No POAF/AFL | P-value* |
| Permanent stroke | 0.68% | 1.72% | 0.00 | 0.39 | - | - | - | - |
| Renal failure with or without dialysis | 11.56% | 12.07% | 11.24% | 0.878 | 9.47% | 14.63% | 5.56% | 0.16 |
| Prolonged ventilation | 5.44% | 12.07% | 1.12% | 0.01 | 7.37% | 9.76% | 5.56% | 0.69 |
| Repeat procedure | - | - | - | - | 1.05% | 2.44% | 0.00% | 0.43 |
| Major complications | 14.97% | 18.97% | 12.36% | 0.27 | 13.68% | 19.51% | 9.26% | 0.15 |
| 30-day operative mortality | 3.40% | 1.72% | 4.49% | 0.65 | 4.21% | 2.44% | 5.56% | 0.63 |
| Mortality/Morbidity composite endpoint (MM) | 15.65% | 18.97% | 13.48% | 0.37 | 15.79% | 19.51% | 12.96% | 0.39 |
| In-hospital death | 3.40% | 1.72% | 4.49% | 0.64 | 4.21% | 2.44% | 5.56% | 0.634 |
| LOS | 10.87 ± 9.51 | 13.43 ± 11.78 | 9.20 ± 7.29 | 0.02 | 9.23 ± 9.47 | 12.49 ± 10.73 | 6.76 ± 7.59 | 0.01 |
| Post-operative days | 9.65 ± 8.73 | 12.38 ± 10.85 | 7.87 ± 6.47 | 0.01 | 7.85 ± 8.94 | 11.20 ± 10.59 | 5.31 ± 6.47 | < 0.01 |
| 30-day readmission | 14.29% | 22.41% | 8.99% | 0.02 | 13.68% | 21.95% | 7.41% | 0.07 |
| AKI | 11.56% | 12.07% | 11.24% | 0.88 | 9.47% | 14.63% | 5.56% | 0.17 |
| Cardiac Arrest | 8.84% | 17.24% | 3.37% | 0.01 | 7.37% | 12.20% | 3.70% | 0.23 |
| Major Bleeding | 6.12% | 8.62% | 4.49% | 0.49 | 3.16% | 2.44% | 3.70% | 1.00 |
| Prosthetic Valve Endocarditis | 2.04% | 0.00% | 3.37% | 0.27 | - | - | - | - |
| TIA | 0.68% | 0.00% | 1.12% | 1.00 | 1.05% | 2.44% | 0.00% | 0.43 |
| MI | 2.72% | 0.00% | 4.49% | 0.15 | 3.16% | 2.44% | 3.70% | 1.00 |
| Major Stroke | 1.36% | 0.00% | 2.25% | 0.51 | 1.05% | 2.44% | 0.00% | 0.42 |
This study’s secondary outcomes included conversion rates (i.e., ViV-TAVR conversion to r-SAVR), all the primary MM composite’s sub-components (i.e., in-hospital death, 30-day death, new perioperative stroke, new renal failure requiring dialysis, extended post-procedural ventilator use, deep sternal wound infection, and/or repeat cardiac procedure), as well as other resource consumption metrics (e.g., total length of stay (LOS), postoperative length of stay (PLOS), and 30-day emergency department visit). Tertiary outcomes included other procedural SAVR/TAVR complications such as acute kidney injury, cardiac arrest, major bleeding, prosthetic valve endocarditis, transient ischemia attack, vascular complications, and myocardial infarction.
To distinguish between procedural complications and patient comorbidities, postoperative complications were defined when patients had no prior history of the complication-related diagnosis within the 2-year period prior to their r-AVR procedure. Based on the “new onset” POAF/AFL definition, therefore, no study patients had preoperative atrial fibrillation or atrial flutter.
Statistical analysis
All analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC). Chi-square tests with exact
In the context of each patient’s unique risk factor profile, patients’ propensity scores were calculated by applying the POAF/AFL multivariable model’s algorithm; the basis for these propensity score calculations was the final POAF/AFL multivariate model that identified patient risk characteristics predictive of POAF/AFL. These patient specific POAF propensity scores were used as model eligible variables for both the MM and READMIT models. To evaluate each model’s predictive power and calibration, the performance metrics (i.e., C-index and Hosmer-Lemeshow test) were reported [Tables 4-6].
Multivariate Model for POAF/AFL
| Variable | OR (95%CI) | P-value |
| Age | 1.05 (1.03-1.07) | < 0.01 |
| Cerebral Vascular Disease | 2.18 (1.05-4.55) | 0.04 |
Multivariate Model for MM
| Variable | OR (95%CI) | P-value |
| Race (Black) | 4.97 (1.61-15.37) | < 0.01 |
| Elixhauser score | 1.05 (1.02-1.08) | < 0.01 |
| POAF/AFL | 1.25 (0.60-2.61) | 0.55 |
Multivariate Model for READMIT
| Variable | OR (95%CI) | P-value |
| POAF/AFL | 3.12 (1.46-6.65) | < 0.01 |
Based on protocol-driven hypotheses, statistical significance was pre-established at P < 0.05 for the
RESULTS
Baseline characteristics of r-SAVR and ViV-TAVR study population
From the SPARCS Database, 74,675 first-time SAVR/TAVR procedures were recorded, of whom 242 patients underwent r-AVR procedures from January 2005 to November 2018 - 147 r-SAVR and 95
Patients who underwent r-SAVR were significantly younger than patients who underwent ViV-TAVR, with a mean age of 61.8 ± 13.9 years and 74.0 ± 11.6 (P < 0.01.), respectively, and are shown in [Table 1]. Across the three major insurance categories (i.e., Commercial insurance, Medicaid/other insurance, and Medicare insurance), the proportions of r_SAVR and ViV-TAVR patients in each category were quite different. Specifically, the rates of Commercial insurance (45.6% r-SAVR versus 22.1% ViV-TAVR), Medicaid/other insurance (4.1% r-SAVR versus 1.1% ViV-TAVR), and Medicare insurance (50.3% r-SAVR versus 76.8% ViV-TAVR) were different (P < 0.01); thus, Medicare coverage rates appeared higher in the ViV-TAVR patients. The ViV-TAVR patients compared to r-SAVR patients were more likely to have the following risk factors: hypertension (85.3% vs. 73.5%, P = 0.03), congestive heart failure (49.5% vs. 18.4%, P < 0.01), cardiomyopathy (13.7% vs. 6.1%, P = 0.05), coronary artery disease (73.7% vs. 28.6%, P < 0.01), chronic obstructive pulmonary disease (25.3% vs. 7.5%, P < 0.01), peripheral vascular disease (9.5% vs. 2.7%,
Baseline characteristics of r-AVR patients with new-onset POAF/AFL
The baseline characteristics of r-AVR patients with and without POAF/AFL are compared [Table 2]. Of the total r-AVR patients, 40.91% of patients experienced post-procedural new onset POAF/AFL. POAF/AFL was present in 39.5% of r-SAVR patients and 43.2% of ViV-TAVR patients (P = 0.57). Patients with POAF/AFL who underwent r-SAVR were older (mean age 69.7 ± 11.1 vs. 56.7 ± 13.2 years, P < 0.01) compared to r-SAVR patients without POAF/AFL. Furthermore, patients with coronary artery disease (39.7% vs. 21.4%, P = 0.02), history of acute myocardial infarction (6.9% vs. 0%, P = 0.02), and chronic kidney disease without dialysis (22.4% vs. 10.1%, P = 0.04) were more frequent in r-SAVR patients with POAF/AFL.
The baseline characteristics of ViV-TAVR patients with and without POAF/AFL are compared [Table 2]. Although ViV-TAVR patients with POAF had higher rates of cerebrovascular disease compared to
Multivariate predictors of POAF/AFL
Multivariable regression analysis of predictors of POAF/AFL are shown in [Table 4]. Older age (OR: 1.05, 95%CI: 1.03-1.07, P < 0.01) and patients with cerebral vascular disease (OR: 2.18, 95%CI: 1.05-4.55, P = 0.04) were significant predictors of POAF/AFL. The c-index of this model was 0.686 and the Hosmer and Lemeshow Goodness-of-Fit Test p-value was 0.07 (i.e., indicating acceptable calibration).
Outcomes of r-SAVR and ViV-TAVR patients with POAF/AFL
The outcomes of r-AVR patients were analyzed and listed by surgery type and POAF/AFL in [Table 3]. Patients who underwent r-SAVR with POAF/AFL, compared to r-SAVR patients without POAF/AFL, were more likely to result in READMIT (22.4% vs. 9.0%, P = 0.02), prolonged ventilation (12.1% vs. 1.1%,
Multivariate predictors of MM and READMIT
For either r-SAVR or ViV-TAVR, the results of the multivariable regression model for MM were reported in [Table 5]. POAF/AFL was not a significant predictor of MM (P = 0.55). With multivariable associations documented (i.e., P < 0.05), however, the variables black race (OR: 4.97, 95%CI: 1.61-15.37, P < 0.01) and Elixhauser score (OR: 1.05, 95%CI: 1.02-1.08, P < 0.01) were associated with increased MM. The c-index of the MM predictive model was 0.713 with the Hosmer and Lemeshow Goodness-of-Fit Test P = 0.85.
As an important resource consumption metric, POAF/AFL (OR: 3.12, 95%CI: 1.46-6.65, P < 0.01) was shown to be a significant predictor of READMIT as shown in [Table 6]. The c-index of this READMIT model was 0.638. Due to the limited number of READMIT final multivariable models’ degrees of freedom, it was not possible to calculate a Hosmer and Lemeshow Goodness-of-Fit test statistic.
DISCUSSION
As a novel contribution to the r-AVR literature, this retrospective observational cohort study documented the impact of POAF/AFL upon clinical outcomes and resource utilization. With an overall POAF/AFL incidence of 41%, this was by far the most common complication found following r-AVR procedures. As noted in the prior literature, older age and cerebral vascular disease were predictors of r-AVR POAF/AFL[16,22-26]. Holding other risk factors constant, POAF/AFL was a predictor of READMIT, but not predictive of MM. Importantly, however, black patients and Elixhauser score were predictors of MM; thus, these “at risk” r-AVR patients deserve future investigation.
The 41% incidence of POAF/AFL in New York State’s SPARCS is consistent with Ejiofor et al.’s study which reported nearly the same rate when comparing postoperative complications between patients who underwent r-SAVR and ViV-TAVR in a matched cohort study[19]. In our study, the incidence of POAF/AFL was not significantly different between r-AVR procedure types; in part, this may be due to the difference in baseline characteristics between patients who underwent r-SAVR and ViV-TAVR.
For first-time AVR procedures, older age was reported as the strongest independent predictor of POAF/AFL; this may be due to structural changes of the heart over time, such as fibrosis and hypertrophy, that affect nodal conduction, and increased comorbidity rates associated with advanced age[16,22-24]. Cerebral vascular disease was also shown to be associated with POAF/AFL as shown in previous studies and is likely an indicator of worse heart health[25,26].
In this New York State’s SPARCS database analysis, patients who underwent ViV-TAVR were older than
Comparing ViV-TAVR average ages (2012 to 2018), however, the r-SAVR patients (mean ± SD; 63.5 ± 13.6) were still substantially younger than the ViV-TAVR patients (mean ± SD; 74.0 ± 11.6). Although ViV-TAVR was a less invasive procedure with lower risks of adverse outcomes compared to r-SAVR, the Food and Drug Administration’s initial ViV-TAVR approval was authorized only for high-risk patients[27-29]. Future research appears warranted to identify if these historical age differences between r-SAVR and Viv-TAVR patients will persist following later FDA approvals for TAVR use in intermediate and lower-risk patient
For first-time AVR procedures, POAF/AFL has been associated with worse mortality, higher rates of stroke, increased length of stay, and readmission[10,16,18,30,31]. Historically, the impact of POAF/AFL in r-AVR patients has not been similarly investigated. For this New York State population, POAF/AFL versus non-POAF/AFL patients did not have different rates of MM or stroke, which may require longer follow-up (beyond 30 days) to observe the full impact of POAF/AFL upon r-AVR patients’ survival. As an important consideration, however, r-AVR patients with POAF/AFL were more likely to be readmitted within 30-days, as well as to have longer hospitalizations. Similarly in a study by Jeong et al. comparing the impact of POAF/AFL in
In this r-AVR study, the MM predictors found were consistent with those previously reported for first-time AVR patients. Black race was documented in this New York Statewide r-AVR population to be predictive of MM; per a prior publication of 991 TAVR patients, this may be due to black race also being associated with a patient-prosthesis mismatch[32]. Based on a study by Taylor et al., black patients who underwent a mitral valve replacement or an AVR were also significantly associated with an increased risk of procedural complications such as prolonged ventilation, longer postoperative stay, and reoperation for bleeding; these patients were generally in poorer health compared to white patients, with higher New York Heart Association class and pulmonary artery pressures and lower ejection fractions[33]. This disparity in health outcomes for black patients is likely due to limited access to care despite these patients residing near hospital centers that perform TAVR, as indicated by Nathan et al. and Bilfinger et al.[34,35]. Elixhauser score, reflecting comorbidity burden in patients undergoing r-AVR procedures was also shown to a predictor of MM and was similarly shown in a study by Nagaraja et al examining 40,604 TAVR patients[36].
CONCLUSION
Affecting 41% of the New York State r-AVR population, the r-AVR POAF/AFL rates are comparable to that of first-time AVR. Due to the increased risk of READMIT for these patients undergoing r-AVR, the “at risk” (i.e., older and cerebrovascular patients undergoing r-SAVR) may now be identified for possible prophylactic treatments such as antiarrhythmic medications post r-AVR procedure. With the increasing volume of SAVR and TAVR procedures being performed, there is also an increasing trend toward bioprosthetic valves and performing ViV-TAVR procedures.
Over time, the r-AVR patients’ profiles have changed. In the latter time periods, there were higher proportions of patients who were older and patients with higher rates of cerebrovascular disease; these patients were “at risk” of readmission within 30 days. Importantly, black patients and/or patients with high Elixhauser comorbidity scores should be proactively identified by clinicians as “at risk” populations. With a focus on targeting these higher-risk r-AVR populations, future clinical trials should investigate innovative prophylaxis and treatment regimens that might improve the clinical outcomes and reduce the differential burden of health care costs incurred. Most importantly, post-r-AVR discharge continuity of care and specialty consultations should be investigated to assure successful convalescence of the POAF/AFL patients.
Limitations
This New York State observational study was limited by its retrospective, cohort study design due to possible unmeasured patient risk factors that may have been confounders impacting this study’s findings. As one potential source of bias, TAVR was initially restricted by the FDA to high-risk patients. Based on revisions in the TAVR eligibility criteria, TAVR was later made available to moderate-risk patients.
With mandatory submissions for all billed encounters enforced by Department of Health audits, this New York statewide database is anticipated to be complete. Given these same billing codes were used in financial transactions by these healthcare facilities with insurance agencies, this database’s findings are most likely highly accurate. Although there is high confidence in the POAF/AFL propensity model, there was data sparsity identified for the MM and READMIT multivariate logistic regression models; using appropriate analytic techniques, however, these models also had relatively high c-indices with no calibration concerns identified; hence, the POAF/AFL association reported with the READMIT endpoint appears to be robust for this New York State-based r-AVR patient population. Although this study’s r-AVR population was smaller in size, the SPARCS database represents the entire New York State population’s experience. Given that r-AVR procedures occur infrequently, future investigations should use regional or national databases to verify the generalizability of these New York State findings.
DECLARATIONS
AcknowledgementsThanks are sent to the Stony Brook University Renaissance School of Medicine’s Department of Surgery Division of Cardiothoracic Surgery staff (Ms. Kathleen O’Brien) and Scholarly Concentrations Programs Research Track staff (Dr. Howard Fleit and Ms. Rhonda Kearns) for their administrative oversight and support. Thanks are also sent to the Office of Quality and Patient Safety in the New York State Department of Health for their SPARCS database access, support and guidance with this project. Finally, our team acknowledges the biostatistical consultation and analytical support provided by the Biostatistical Consulting Core (Dr. Jie Yang and Ms. Xiaoyue Zhang) at the Renaissance School of Medicine, Stony Brook University.
Authors’ contributionsSubstantive intellectual contribution: Dokko J, Novotny S, Kolba N, Agha S, Yaligar A, Tummala V, Parikh PB, Pyor AD, Tannous HJ, Shroyer AL, Bilfinger T
Study conception and design: Dokko J, Novotny S, Tannous HJ, Shroyer AL, Bilfinger T
Data coding/acquisition: Dokko J, Novotny S, Kolba N, Tummala V, Agha S, Yaligar A, Parikh PB, Pryor AD, Bilfinger T, Shroyer AL, Bilfinger T
Data analysis/interpretation: Dokko J, Shroyer AL, Bilfinger T
Writing the initial abstract/manuscript: Dokko J, Novotny S, Shroyer AL, Bilfinger T
Revising the submitted abstract/manuscript: Dokko J, Novotny S, Kolba N, Agha S, Yaligar A, Tummala V, Parikh PB, Pryor AD, Tannous HJ, Shroyer AL, Bilfinger T
Reviewing and approving the submitted abstract/manuscript: Dokko J, Novotny S, Kolba N, Agha S, Yaligar A, Tummala V, Parikh PB, Pryor AD, Tannous HJ, Shroyer AL, Bilfinger T
Availability of data and materialsAll patient records and data were extracted from 2005-2018 SPARCS Database. Due to contractual limitations (i.e., concerns regarding patient records being re-identified) in the Stony Brook University data use agreement, no reports could contain cells that had lower than 10 events; thus, only percentages were reported in [Tables 1-3].
Financial support and sponsorshipThis study was supported by the Stony Brook Renaissance School of Medicine Department of Medicine small grant (led by Dr. Puja B. Parikh and Dr. A. Laurie Shroyer) and the Division of Cardiothoracic surgery General T.F. Cheng endowment led by Dr. Henry J. Tannous.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThis study was approved for IRB exemption under not human study research on 11/23/2021 [IRB2021-00605: POAF r-AVR].
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Hu PP. TAVR and SAVR: current treatment of aortic stenosis. Clin Med Insights Cardiol 2012;6:125-39.
2. Saad AM, Kassis N, Isogai T, et al. Trends in outcomes of transcatheter and surgical aortic valve replacement in the United States (2012-2017). Am J Cardiol 2021;141:79-85.
3. Mori M, Gupta A, Wang Y, et al. Trends in transcatheter and surgical aortic valve replacement among older adults in the United States. J Am Coll Cardiol 2021;78:2161-72.
4. Bartus K, Sadowski J, Litwinowicz R, et al. Changing trends in aortic valve procedures over the past ten years-from mechanical prosthesis via stented bioprosthesis to TAVI procedures-analysis of 50,846 aortic valve cases based on a Polish National Cardiac Surgery Database. J Thorac Dis 2019;11:2340-9.
5. Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol 2020;76:2492-516.
6. Björn R, Nissinen M, Lehto J, et al. Late incidence and recurrence of new-onset atrial fibrillation after isolated surgical aortic valve replacement. J Thorac Cardiovasc Surg 2021:S0022-5223(21)00587.
7. Kostyunin AE, Yuzhalin AE, Rezvova MA, Ovcharenko EA, Glushkova TV, Kutikhin AG. Degeneration of bioprosthetic heart valves: update 2020. J Am Heart Assoc 2020;9:e018506.
8. Herold J, Herold-Vlanti V, Sherif M, et al. Analysis of cardiovascular mortality, bleeding, vascular and cerebrovascular events in patients with atrial fibrillation vs. sinus rhythm undergoing transfemoral Transcatheter Aortic Valve Implantation (TAVR). BMC Cardiovasc Disord 2017;17:298.
9. Mentias A, Saad M, Girotra S, et al. Impact of pre-existing and new-onset atrial fibrillation on outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019;12:2119-29.
10. Kalra R, Patel N, Doshi R, Arora G, Arora P. Evaluation of the incidence of new-onset atrial fibrillation after aortic valve replacement. JAMA Intern Med 2019;179:1122-30.
11. Indja B, Woldendorp K, Vallely MP, Grieve SM. New onset atrial fibrillation following transcatheter and surgical aortic valve replacement: a systematic review and Meta-analysis. Heart Lung Circ 2020;29:1542-53.
12. Motloch LJ, Reda S, Rottlaender D, et al. Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg 2012;93:124-31.
13. Tanawuttiwat T, O'Neill BP, Cohen MG, et al. New-onset atrial fibrillation after aortic valve replacement: comparison of transfemoral, transapical, transaortic, and surgical approaches. J Am Coll Cardiol 2014;63:1510-9.
14. Vora AN, Dai D, Matsuoka R, et al. Incidence, management, and associated clinical outcomes of new-onset atrial fibrillation following transcatheter aortic valve replacement: an analysis from the STS/ACC TVT registry. JACC Cardiovasc Interv 2018;11:1746-56.
15. Saxena S. A review of the incidence and management of new-onset atrial fibrillation post transcatheter aortic valve implantation. AJBSR 2020;8:117-21.
16. Tarantini G, Mojoli M, Windecker S, et al. Prevalence and impact of atrial fibrillation in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement: an analysis from the SOURCE XT prospective multicenter registry. JACC Cardiovasc Interv 2016;9:937-46.
17. Ding Y, Wan M, Zhang H, Wang C, Dai Z. Comparison of postprocedural new-onset atrial fibrillation between transcatheter and surgical aortic valve replacement: a systematic review and meta-analysis based on 16 randomized controlled trials. Medicine (Baltimore) 2021;100:e26613.
18. Shahim B, Malaisrie SC, George I, et al. Postoperative atrial fibrillation or flutter following transcatheter or surgical aortic valve replacement: PARTNER 3 trial. JACC Cardiovasc Interv 2021;14:1565-74.
19. Ejiofor JI, Yammine M, Harloff MT, et al. Reoperative surgical aortic valve replacement versus transcatheter valve-in-valve replacement for degenerated bioprosthetic aortic valves. Ann Thorac Surg 2016;102:1452-8.
20. New York State Department of Health. Vital Statistics of New York State 2018. Published 2018. Accessed January 24, 2022.
21. New York State Department of Health. Statewide Planning and Research Cooperative System (SPARCS). Accessed 2021.
22. Axtell AL, Moonsamy P, Melnitchouk S, et al. Preoperative predictors of new-onset prolonged atrial fibrillation after surgical aortic valve replacement. J Thorac Cardiovasc Surg 2020;159:1407-14.
23. Amar D, Zhang H, Leung DH, Roistacher N, Kadish AH. Older age is the strongest predictor of postoperative atrial fibrillation. Anesthesiology 2002;96:352-6.
24. Auer J, Weber T, Berent R, Ng CK, Lamm G, Eber B. Risk factors of postoperative atrial fibrillation after cardiac surgery. J Card Surg 2005;20:425-31.
25. Mariscalco G, Biancari F, Zanobini M, et al. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. J Am Heart Assoc 2014;3:e000752.
26. Mortazavi SH, Oraii A, Goodarzynejad H, et al. Utility of the CHA2DS2-VASc score in prediction of postoperative atrial fibrillation after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2022;36:1304-9.
27. Mahmaljy H, Tawney A, Young M. Transcatheter Aortic Valve Replacement. 2022 May 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
28. Murdoch DJ, Webb JG. Transcatheter valve-in-valve implantation for degenerated surgical bioprostheses. J Thorac Dis 2018;10:S3573-7.
29. Malik AH, Yandrapalli S, Zaid S, et al. Valve-in-valve transcatheter implantation versus redo surgical aortic valve replacement. Am J Cardiol 2020;125:1378-84.
30. Jeong HK, Yoon N, Kim JH, et al. Post-operative atrial fibrillation impacts on outcomes in transcatheter and surgical aortic valve replacement. Front Cardiovasc Med 2021;8:789548.
31. Biviano AB, Nazif T, Dizon J, et al. Atrial fibrillation is associated with increased mortality in patients undergoing transcatheter aortic valve replacement: insights from the Placement of Aortic Transcatheter Valve (PARTNER) trial. Circ Cardiovasc Interv 2016;9:e002766.
32. Stamou SC, Chen K, James TM, et al. Predictors and outcomes of patient-prosthesis mismatch after transcatheter aortic valve replacement. J Card Surg 2020;35:360-6.
33. Taylor NE, O’Brien S, Edwards FH, Peterson ED, Bridges CR. Relationship between race and mortality and morbidity after valve replacement surgery. Circulation 2005;111:1305-12.
34. Nathan AS, Yang L, Yang N, et al. Racial, ethnic, and socioeconomic disparities in access to transcatheter aortic valve replacement within major metropolitan areas. JAMA Cardiol 2022;7:150-7.
35. Bilfinger T, Nemesure A, Pyo R, et al. Distressed communities index in patients undergoing transcatheter aortic valve implantation in an affluent county in New York. J Interv Cardiol 2021;2021:8837644.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Dokko J, Novotny S, Kolba N, Agha S, Yaligar A, Tummala V, Parikh PB, Pryor AD, Tannous HJ, Shroyer AL, Bilfinger T. Predictors and risk-adjusted outcomes of new-onset postoperative atrial fibrillation in repeat surgical and valve-in-valve transcatheter aortic valve replacement . Vessel Plus 2022;6:53. http://dx.doi.org/10.20517/2574-1209.2022.11
AMA Style
Dokko J, Novotny S, Kolba N, Agha S, Yaligar A, Tummala V, Parikh PB, Pryor AD, Tannous HJ, Shroyer AL, Bilfinger T. Predictors and risk-adjusted outcomes of new-onset postoperative atrial fibrillation in repeat surgical and valve-in-valve transcatheter aortic valve replacement . Vessel Plus. 2022; 6: 53. http://dx.doi.org/10.20517/2574-1209.2022.11
Chicago/Turabian Style
Dokko, Julia, Samantha Novotny, Natalie Kolba, Sohaib Agha, Ashutosh Yaligar, Vineet Tummala, Puja B. Parikh, Aurora D. Pryor, Henry J. Tannous, A. Laurie Shroyer, Thomas Bilfinger. 2022. "Predictors and risk-adjusted outcomes of new-onset postoperative atrial fibrillation in repeat surgical and valve-in-valve transcatheter aortic valve replacement " Vessel Plus. 6: 53. http://dx.doi.org/10.20517/2574-1209.2022.11
ACS Style
Dokko, J.; Novotny S.; Kolba N.; Agha S.; Yaligar A.; Tummala V.; Parikh PB.; Pryor AD.; Tannous HJ.; Shroyer AL.; Bilfinger T. Predictors and risk-adjusted outcomes of new-onset postoperative atrial fibrillation in repeat surgical and valve-in-valve transcatheter aortic valve replacement . Vessel Plus. 2022, 6, 53. http://dx.doi.org/10.20517/2574-1209.2022.11
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.