Perspective on the development of a bioengineered patch to treat heart failure: rationale and proposed design of phase I clinical trial
Abstract
This perspective focuses on the development of tissue engineered (TE) cell-based therapies to treat left ventricular (LV) dysfunction and chronic heart failure (CHF). The development of induced pluripotent stem cells enabled investigators to seed or co-culture human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) alone and in combination with other cells onto bioengineered scaffolds applied to the epicardial surface of the damaged left ventricle. Using our work as an example, we show how a xenograft implant of a bioengineered scaffold embedded with human neonatal fibroblasts and seeded with hiPSC-CMs partially reversed maladaptive LV remodeling and improved LV systolic/diastolic function in an immune-competent rat model of CHF. The fibroblasts lay down an extracellular matrix and secrete growth factors that increase myocardial blood flow. This approach provides an improved cell payload that covers a larger area of the damaged left ventricle as opposed to direct cell injections into the heart or down the coronary arteries. These studies combined with ongoing studies in immune-competent Yucatan mini swine treated with the same xenograft led to the preliminary design of a proposed Phase I clinical trial that will be presented to the Federal Drug Administration. For the proposed Phase I clinical, this TE patch will be implanted onto the epicardial surface of non-immunosuppressed patients undergoing elective Coronary Artery Bypass Grafting with Ejection Fractions ≥ 20% and ≤ 45%. The primary endpoints will be adverse events/severe adverse events associated with placing the TE patch on the heart. While Phase I trials are primarily safety trials, this proposed trial is designed to obtain some potential efficacy endpoints to help with the design of future Phase II/III clinical trials. These endpoints include changes in LV remodeling that were seen in the pre-clinical animal models as well as including endpoints that focus on patient well-being.
Keywords
INTRODUCTION
There is an urgent unmet need to develop new treatments for chronic heart failure (CHF) due to approximately 26 million cases worldwide with an increasing prevalence[1]. The economic burden limits the options for treatment of all stages of CHF, particularly late-stage CHF, where mechanical assist devices and heart transplantation are the only options. Regenerative medicine has the potential to address this issue by improving cardiac function and, importantly, improving quality and increased length of life in patients with CHF. The initial development of regenerative approaches to treat CHF with intra-myocardial injections or intracoronary infusions had limited efficacy but importantly have been shown to be safe. While there are some data showing benefit with direct injections into the heart[2-5], most investigators believe that the lack of benefit from direct injections of cell-based therapy is due to injected cells not surviving transplantation or not persisting long enough to generate a functional benefit because of (1) lack of matrix/structural support; (2) limited nutrient/blood supply to the infarcted myocardium; (3) loss of cells due to washing out through injection sites, or a combination of all of these. To address these issues, investigators have developed tissue engineering (TE) approaches that include hydrogel base gels, cell sheets, 3-dimensional printing patches, and others[6-13]. This review outlines the development of TE approaches to the treatment of CHF, starting with an acellular collagen patch advancing to a TE patch embedded with human neonatal fibroblasts and seeded with human cardiomyocytes. This perspective summarizes the pre-clinical animal work in our laboratory and others, showing the progression from rodent to large animal models of ischemic cardiomyopathy. Lastly, it includes the rationale and design of a proposed Phase I clinical trial that will be submitted to the Food and Drug Administration (FDA) to study a bioengineered patch to treat patients with poor LV function.
Background
Induced pluripotent stem cells
In 2012, the Nobel Prize in Medicine or Physiology was awarded to John B. Gurdon and Shinya Yamanaka for the discovery that mature cells can be reprogrammed to become pluripotent, induced pluripotent stem cells (iPSCs)[14]. This discovery led to the directed differentiation of iPSCs into cardiomyocytes by reprogramming adult somatic cells into iPSCs and differentiating these into cardiomyocytes, i.e., human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). This resulted in high-quality hiPSC-CMs available in unlimited quantities for use in the laboratory and the clinic, accepting that making these cells at scale is still a costly endeavor and maybe a limitation as a cost of goods.
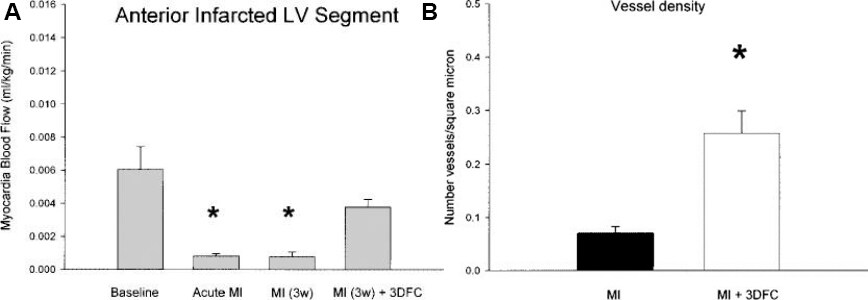
Our laboratory began working on TE approaches to treat CHF in 2006 utilizing a simple acellular 3-dimensional collagen patch applied in a rat model of non-transmural cryoinjury[15]. Next in the development was using a VICRYL® (Ethicon, USA) (polyglactin 910) biodegradable patch seeded with neonatal human fibroblasts in infarcted rats[7]. This fibroblast patch improved cardiac function and increased myocardial blood flow to the infarcted anterior wall of the left ventricle in rats after an acute myocardial infarction (MI) created by ligation of the left coronary artery. While these results were intriguing, the clinical need was a treatment for CHF, not to treat an acute MI. Importantly, there are major differences in the myocardial substrate that occurs with an acute MI compared to CHF. Inflammatory responses associated with an acute MI and the accompanying “homing” signal are believed to set up a milieu that makes treating an acute MI easier than CHF. This is an important point because positive studies of cell-based therapy in animal models of acute MI do not seem to translate into the same benefits in animal models of CHF. This proved true in our work when the VICRYL® patch with the embedded fibroblasts was implanted on the infarcted left ventricular (LV) wall of rats 3 weeks after MI, at which time the rats were in CHF, myocardial blood flow and microvessel density increased in the infarcted anterior wall, but LV function did not improve[7]
Figure 1. (A). Anterior wall myocardial blood flow in sham (N = 11), at the time of acute myocardial infarction (MI, N = 7), MI at 3 weeks (N = 4), and MI at 3 weeks with 3DFC (N = 4). Note that the 3-dimensional fibroblast construct (3DFC) improved blood flow in the infarcted wall. Values are mean ± SE; *P < 0.05 versus baseline and MI (3w) + 3DFC[7]. Cell Transplantation 2009;3:283-296. (B). Vessel density defined by Factor VIII staining. Note the increase in vessel density in the area with the 3-dimensional fibroblast construct (3DFC) compared to the untreated myocardial infarction (MI). MI (N = 9), MI + 3DFC (N = 8). Values are mean ± SE. *P < 0.05 versus MI[7].
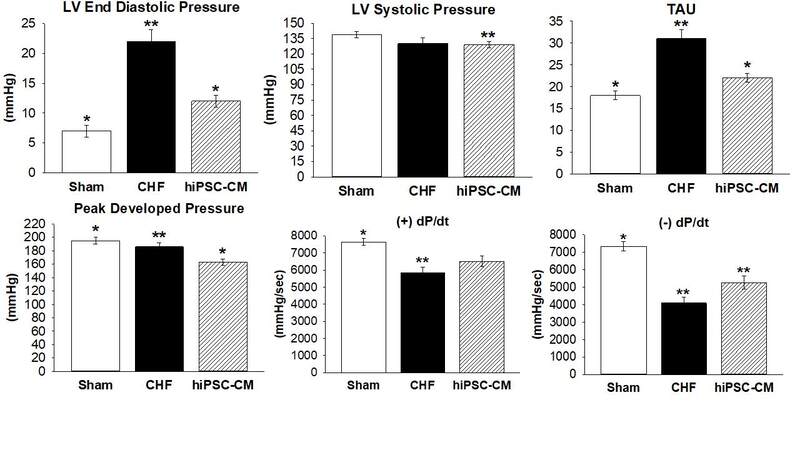
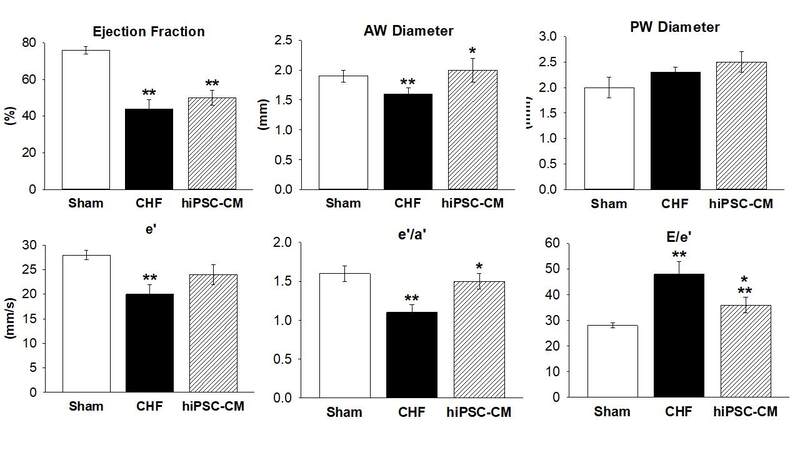
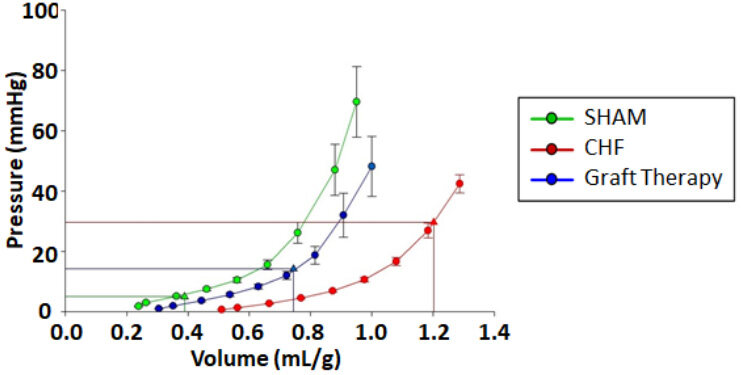
Our laboratory utilized the development of iPSCs by seeding hiPSC-CMs onto our fibroblast embedded patch. Thus, we had a human patch containing a “cell payload” that we could test in animal models of CHF and eventually use in patients[16]. Our work showed that the combination of fibroblasts and cardiomyocytes improved cardiac function in rats with CHF. The important findings of this study were that the TE patch-treated rats decreased LV-end diastolic pressure and the time constant of LV relaxation (Tau), increased anterior wall thickness in diastole, improved echocardiographic-derived indices of diastolic function E/e’ [ratio of early peak flow velocity to early peak LV velocity] and e’/a’ [ratio of early to late peak LV velocity]. The graft-treated rats have a pressure/volume filling curve shifted back towards SHAM and a lower LV end-diastolic operating volume, consistent with reversal of maladaptive remodeling related to compliance[16-18]
Figure 2. Hemodynamics-Comparison CHF rats treated with hiPSC-CM patch; Data are mean ± SE @ 6 weeks after implantation or sham thoracotomy where *denotes significance (P < 0.05) vs. CHF, **denotes significance (P < 0.05) vs Sham. Sham, N = 12; CHF, N = 8-21, hiPSC-CM, N = 20-24 per group. CHF: Chronic heart Failure; hiPSC-CM: human induced pluripotent stem cell derived cardiomyocytes; LV Sys P: systolic pressure; LV EDP: end diastolic pressure; Tau: time constant of relaxation constant; LV dP/dt: change in LV pressure over change in time; PDP: peaked-developed pressure[16]; LV: left ventricular.
Figure 3. Echocardiographic parameters-CHF rats treated with hiPSC-CM patch; Data are mean ± SE where *denotes significance (P < 0.05) vs. CHF, and **denotes significance (P < 0.05) vs. Sham. Sham: N = 12; CHF: N = 8-21, hiSPC-CM: N = 20-24 per group. CHF: Chronic heart failure; hiPSC-CM: human induced pluripotent stem cell derived cardiomyocytes, EF: ejection fraction; e’: early LV relaxation velocity; E/e’: ratio of early peak flow velocity to early peak LV velocity; e’/a’: ratio of early to late peak LV velocities; AWdia: anterior wall thickness in diastole; PWdia: posterior wall thickness in diastole[16]; LV: left ventricular.
Figure 4. : Rat Diastolic Pressure-Volume Relationship; Ex vivo Pressure-Volume (P/V) diastolic filling curves of SHAM (n = 4), CHF (n = 5), and graft-treated rats (n = 7). Left ventricular volumes were normalized to each rat’s body weight. CHF results in a rightward shift in the PV filling curve and an increase in the operating volume. The graft-treated rats have a P/V filling curve shifted back towards SHAM and a lower end operating volume, consistent with reversal of maladaptive remodeling related to compliance[18].
All rats remained in normal sinus rhythm, and no dysrhythmias were documented. Rats treated with the patch showed improved electrical activity with faster conduction through the area of the scar [Figure 5], increased LV wall thickness with evidence of preserved or rejuvenated cardiac tissue [Figure 6]. All rats remained in sinus rhythm with synchronized contraction through the patch area documented by transthoracic echocardiography. Transplanted hiPSC-CMs were observed at 7 days but not at 21 days after implantation; despite this LV function improved[16,18]. The patch increased gene expression of vascular endothelial growth factor, angiopoietin 1, gap junction a-1 protein (connexin 43), β-myosin heavy chain 7, and insulin growth factor-1 expression in the infarcted heart after treatment[16]. In more recent work, we have characterized the hiPSC-CMs in-vitro prior to implantation by defining their responses to adrenergic stimulation. Because we are dealing with a xenograft transplant, i.e., putting human fibroblasts and human cardiomyocytes into an immune-competent rat, we studied the immune responses of the TE patch in vivo with the rats receiving no immune suppression. We showed that our TE patch partially reversed LV remodeling[18]. This work documented the therapeutic benefits of our bioengineered patch in rats with CHF. We then pursued a swine model that more closely relates to CHF patient populations and future clinical products.
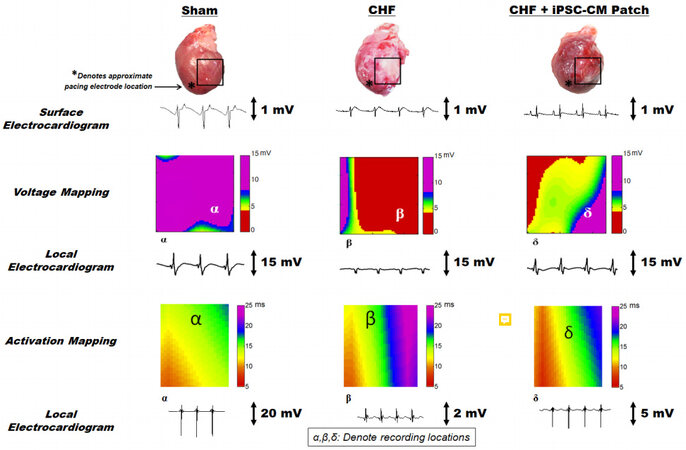
Figure 5. In vivo electrophysiological analyses confirm human induced pluripotent stem cell derived cardiomyocyte (hiPSC-CM) electromechanical enhancement of the host myocardium. Voltage and activation mapping were performed using a custom-engineered software program. Asterisks indicate the approximate epicardial pacing location for activation mapping only. Boxes on the sham, chronic heart failure (CHF), and CHF + hiPSC-CM patch hearts denote tissue region mapped to generate color maps for voltage and activation mapping. The α, β, and δ denote the focal area of local electrocardiograms from each group with respect to voltage or activation maps[16]. *Denotes the approximate location of pacing electrode.
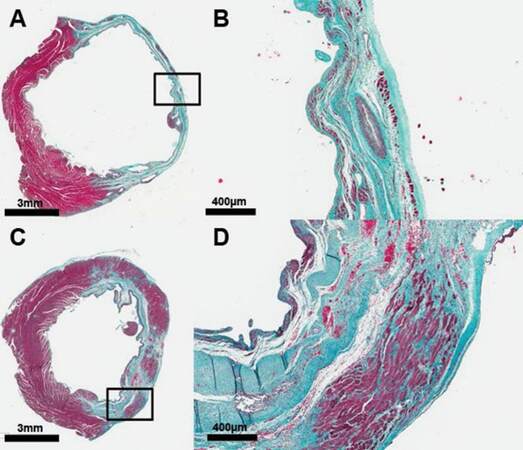
Figure 6. The human induced pluripotent stem cell derived cardiomyocyte (hiPSC-CM) patch increases left ventricular (LV) wall thickness and preserves or regenerates myocardium, or both. The Mason trichrome-stained LV cross-sections of (A, B) 6-week chronic heart failure (CHF) control rats with an infarct but no treatment, and (C, D) hiPSC-CM patch-treated CHF rats 6 weeks after coronary artery ligation (3 weeks after implantation). Healthy myocardium is represented as red-purple, collagen/scar as blue, and red blood cells as small red dots. Box insets represent an area of higher magnification. Patch treatments were single dose in all animals regardless of infarct size. The dose was based on the size of scaffold to cover most of the anterior surface of the left ventricle. Placement of the patch increases anterior wall thickness and myocyte density in the previously infarcted region[16].
Composition of bioengineered patch
The bioengineered patch is composed of a biodegradable mesh, embedded with human neonatal fibroblasts, and seeded with hiPSC-CMs. We have focused on biodegradable meshes where the chemical compounds have supported 510K cleared products.
Human induced pluripotent stem cells derived cardiomyocytes
The hiPSC-CMs are differentiated from clinical-grade human iPSCs obtained from an eligible donor. While it is well recognized that hiPSC-CMs are considered to be immature, our work shows that even in their “immature” state, these cells are able to improve LV function in animal models of CHF[16]. In this application, it may be because hiPSC-CMs potentially exhibit more plasticity, thus allowing them to more easily seed on the patch and secrete paracrine factors that are thought to be part of the mechanism(s) of action of this cell-based therapy. The human neonatal dermal fibroblasts (from neonatal foreskin discards) are obtained from a working cell bank and expanded for patch fabrication with a cardiomyocyte-to-fibroblast ratio of 1:1 to 2:1[16]. For rat studies, the approximately 1.6 cm diameter patches were generated; for the swine studies, 5.0 cm diameter, large enough to cover the infarcted area of the left ventricle. Tissue patches were cultured for up to 30 days to achieve full thickness confluent patches.
Comparison of our work with that of other investigators
Work by our group and other investigators has shown that implanting TE scaffolds and injecting cells in pre-clinical animal models of heart failure has proven safe. Although the types of cells differ and the methods of administering cells are different in these studies, there has been no evidence of acute pathologic tissue or cell rejection, and there have been no reports of teratoma formation[19]. Like our work, data from other investigators generally show hemodynamic benefit, attenuation of LV remodeling but limited long-term engraftment even with concomitant immune suppression[13]. There are reports of embryonic stem cell injections into the heart with patches into non-human primates showing induction of ventricular arrhythmias[2]. There are also earlier studies of injecting skeletal myoblasts into patients with poor LV function who developed ventricular arrhythmias and required placement of Intracardiac Cardiac Defibrillators[20]. Other investigators have used biocompatible polyelectrolyte (PE) complexes in which oppositely charged particles are employed within a structure can be used to develop structures to support organized architecture. The PE complex can be in various arrangements including polymer to polymer, or polymer to cell, and can be used to engineer stratified structures or functional subunits within a larger structure. They can also be used to enhance interactions between therapeutic and host tissues. While PE complexes have been described in cardiac therapeutic applications, it is unknown if they provide extensive benefit over other approaches that are simpler to manufacture, especially when the scale is important[21-23]. We were successful in creating similar architecture with our TE scaffold.
We have not seen any ventricular arrhythmias in our rat studies or in our ongoing swine studies. All animal work was performed under the oversight of the University of Arizona Institutional Animal Care and Use Committee (IACUC) under PHS Animal Welfare Assurance Number: D16-00159 (A3248-01), USDA Animal Research Facility Registration Number and AAALACi Number 000163. The IACUC oversees the University of Arizona’s research program and is responsible for reviewing and approving all activities utilizing vertebrate animals for research, ensuring compliance with federal animal welfare regulations, inspecting animal facilities and investigator laboratories, investigating animal concerns, and overseeing training and educational programs.
In human studies, embryonic stem cell-derived cardiovascular progenitor cells have been injected in a pilot study of 6 patients with decreased LV function and showed that the treatment was safe. No arrhythmias were noted by intra-cardiac defibrillator (ICD) interrogations, and no tumors were reported[12]. Based on this work, these investigators performed the second trial of 10 patients, but these results are still forthcoming. A clinical trial has commenced using PSC-CMs to treat patients in CHF and is currently ongoing in Japan [NCT04696328]. Cardiothoracic surgeons are implanting cell sheets on the epicardial surface of the heart in immune-suppressed patients. All these studies are referenced in our previous publication[19].
The most relevant study as a comparison to our work is the clinical trial in Japan with cell sheets. While this trial is ongoing, the potential commercial success and adoption of these technologies may be limited because these cell sheets are reported to be friable and difficult to handle. Our patch addresses these issues specifically: our patch is robust, easily handled by the surgeons, can be cryopreserved, and does not require any specifical technical skills to implant the patch. Lastly, we have successfully implanted the patch using minimally invasive thoracoscopic and robotic approaches (unpublished data).
OUTLINE OF STUDY OF TISSUE ENGINEERED PATCH IN YUCATAN MINI SWINE
For the clinical development of our TE patch, we are implanting it in a large animal model of MI-induced CHF. The study is currently ongoing. In brief, Yucatan mini swine 10-20 months of age undergo a 90-minute balloon occlusion/reperfusion of the left anterior descending (LAD) coronary artery and recover for 4 weeks to allow for heart failure to develop. Four weeks after MI, a mini sternotomy is performed, and a single 5.0 cm diameter TE patch is sutured onto the epicardial surface of the infarcted anterior wall of the left ventricle. Swine are randomized to TE patch or a mesh only control group. The mesh only control group receives an inert biomaterial without cells. All study personnel are blinded to the treatment groups. A manuscript from our laboratory outlines the specific details of this model using cardiac magnetic resonance (CMR) imaging to define the extent of scar area in the left ventricle 1-month after MI[24].
The following studies are performed in these animals
Hemodynamics & pressure/volume loops
At baseline, 1-month, and 6-months after MI, we performed left and right heart catheterizations using Millar Ventri-Cath pressure-volume loop catheter® (Houston, TX, USA)® solid-state catheters to obtain measurements of LV pressures, LV end-systolic elastance and end-diastolic pressure/volume measurements and Swan Ganz catheters to obtain right heart pressures and measure cardiac output.
Cardiac magnetic resonance (CMR) imaging
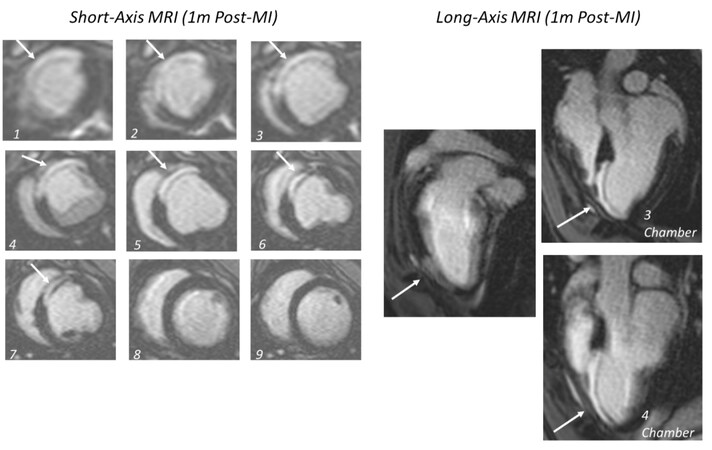
We obtained CMR imaging at baseline, 1-month, 2-months, 4-months and 7-months after MI to obtain measurements of LV volume, LV mass, and scar size. We use the CMR image obtained at 1-month after MI to define the scar’s location to help guide the surgeon on where to place the TE patch [Figure 7][24]. We found this is important because the infarct area routinely extends beyond the visual assessment of the scar area seen in the operating room.
Arrhythmia monitoring by internal cardiac monitors (ICMs)
All swine have an ICM Reveal Linq (Medtronic) loop recorder placed one month prior to the MI procedure to monitor baseline arrhythmias. The ICM is implanted subcutaneously along the left scapula. These recorders are left in place for the duration of the study and interrogated every 4 weeks, or weekly if the battery is low. An independent electrophysiology nurse and an electrophysiologist review abnormal rhythms and separate true abnormal rhythms from the artifact.
Quality of life activity monitoring
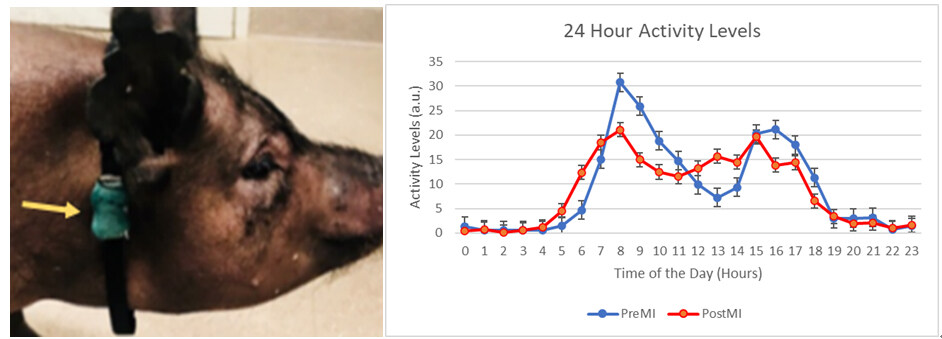
Each animal was fitted with a collar that contains a FitBark™ activity tracker ([Figure 8] Left panel). Collars were placed on the animals 2-4 weeks prior to creating the MI to acquire baseline data. Data are retrieved from the collars every 24-72 hours and remain on for the entire 6-month duration of the study. The FitBark collars acquire “activity units”, an arbitrary unit of measure assigned by the manufacturer to quantify activity levels that show changes in activity as a surrogate for quality of life ([Figure 8] Right panel).
Figure 8. (Left) Yucatan Mini Swine fitted with a FitBark 2 (yellow arrow) on a nylon collar. (Right) The activity data generated by the FitBark collars are a proxy for total physical activity of the swine. FitBark uses 3-dimensional accelerometer readings to generate their arbitrary units (a.u.) to measure the activity levels of the swine. Accelerometer readings are taken multiple times per second and integrated over a 1-minute interval.
Treadmill activity
We trained the swine to walk on treadmills using treats as a stimulus [Figure 9]. For the first minute, swine are started at 1 mph and steadily increased to a maximum rate of 2.1-2.7 mph is reached and maintained for 5 minutes. During the treadmill walks, the swine are closely monitored by technicians to ensure their safety. Heart rate data is collected before, immediately after, and 5 minutes after testing using a stethoscope.
As noted above, these studies are ongoing and blinded to the laboratory personnel. As soon as data from these studies are available, they will be presented to the FDA as part of our Pre-IND proposal.
CLINICAL DEVELOPMENT OF TE PATCH
The TE patch is a single-entity biologic and device combination product reviewed by the Center for Biologics Evaluation and Research (CBER) within the FDA. As part of the clinical evaluation process for the TE patch, we had an INitial Targeted Engagement for Regulatory Advice on CBER products (INTERACT) meeting. This INTERACT meeting is an informal nonbinding consultation with CBER that enables us to “obtain preliminary informal consultation for innovative investigational products at an early stage of development on issues that are not yet at the pre-IND meeting phase.” This is intended for innovative investigational products that introduce unique challenges due to the unknown safety profiles resulting from the use of complex manufacturing technologies, the development of innovative devices, or cutting-edge testing methodologies. This INTERACT meeting helped refine our non-clinical development plans and clinical protocols to test TE patch in Phase I clinical trial.
MATERIALS AND METHODS
Rationale and design of phase I clinical trial
In the Phase I trial, we propose to implant the TE patch on the epicardial surface of the heart in patients undergoing elective on-pump coronary-artery bypass grafting (CABG) via median sternotomy who have poor LV function. The choice of this CABG patient population allows for TE patch implantation with minimal additional surgical risk since the epicardium will already be exposed during the CABG procedure. The TE patch will be implanted directly on the epicardium over the diseased tissue based on the location of the infarcted area defined by CMR and sutured in place. Implantation of the TE patch will not affect the standard of care for the CABG or any subsequent procedures that may be necessary to manage progressive heart failure. Drug regimens will remain standard for CABG. Implantation of the TE patch will not require additional medications such as immunosuppression. For informational purposes, human leukocyte antigen antibody titers will be collected from treated patients. We discussed the development of this protocol with about 30 cardiologists and cardiothoracic surgeons, and there was uniform agreement in choosing this patient population.
Clinical protocol for phase I trial of TE patch
Study Title: An open-label Phase I clinical trial to evaluate the safety and feasibility of an allogeneic human cardiac tissue graft for treatment of chronic heart failure.
Overview of study design: The objective of this proposed Phase I study is to assess the safety and feasibility of this TE patch treatment in 12 patients with decreased LV function [ejection fraction (EF) ≥ 20% and ≤ 45%] resulting from previous ischemic damage, who will be undergoing elective, isolated, on-pump CABG to be completed via median sternotomy. Data will be acquired to assess adverse events (AEs) and serious adverse events (SAEs) in a treatment and a chart review control group [Tables 1 and 2].
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| • The subject is older than 18 years of age; both men and women will be enrolled. • The subject must be referred for elective isolated CABG to occur on cardiopulmonary bypass via median sternotomy. • The left ventricular ejection fraction (LVEF) must be ≥ 20% and ≤ 45% (as determined via echocardiography). • The subject must be able to provide informed consent and follow-up with protocol procedures. • The subject must have the ability to undergo an MRI and tolerate imaging contrast agents. • If the subject is a woman capable of childbearing, she must agree to use a reliable method of contraception for the duration of participation. • The subject must be ambulatory and able and willing to complete a six-minute walk unassisted. | • Active inflammatory or autoimmune disease, on immunosuppression. • Pregnant or lactating. • Participation in another clinical study unless permission given from PI or Co-I. • History of or known chronic pericarditis. • Frequent, recurrent clinically significant ventricular tachycardia within 30 days prior to CABG procedure. • Previous heart valve replacement, including transcatheter aortic valve replacement (TAVR). • Cerebrovascular event/stroke or documented transient ischemic attack (TIA) within the one year prior to enrollment. • Major trauma (as determined by study PI or Co-I) within 14 days prior to enrollment. • Previous heart surgery, but previous PCI is acceptable. • Implanted cardioverter defibrillator (ICD) positioning that prohibits adequate analysis of required measurements via MRI (as assessed with baseline MRI). Note: a patient may have an ICD implanted after CABG surgery; this will not disqualify the patient. • Body mass index (BMI)greater than 40. • If a patient has diabetes, A1C above 8 at last time of measure. • The subject is on oxygen therapy and/or has an intrinsic lung disease that could compromise post-operative recovery. • Calculated glomerular filtration rate (GFR) less than 30. • Previous negative reaction or allergy to materials used for hernia repair. • Known bleeding disorder that could cause surgical complications. • Restrictive Cardiac Physiology defined by CMR. • History of autoimmune disease and/or any form of immune suppressant therapy, steroid, within the past three months. • Known family history of hypertrophic cardiomyopathy. • Systolic pulmonary artery (PA) pressure of 45 or above assessed by transthoracic echocardiography. • Life expectancy of less than one year. |
Time of assessments in treatment group
| Study procedure | Enrollment screening | Baseline | Intra OP | Prior to discharge | 1 month | 3 months | 12 months |
| Informed consent | x | ||||||
| Chart review | x | x | x | x | X | ||
| Cardiopulmonary Exam* | x | x | x | x | X | ||
| Echo** | x | x | X | ||||
| Cardiac MRI | x | x | X | ||||
| Routine labs (as defined by the study site) | x | x | |||||
| Biomarkers (NT-proBNP, hsCRP, hsTroponin) | x | x | x | X | |||
| Immunogenicity markers (HLA antibodies) | x | x | x | X | |||
| Rhythm analysis*** | x | x | |||||
| NYHA class | x | x | X | ||||
| Six-minute walk | x | x | X | ||||
| Quality of life questionnaires (KCCQ, SAQ) | x | x | X | ||||
| SAE/AE assessment | x | x | x | x | X | ||
| Blood banking | x | x | x | x | X | ||
| Operative data | x |
The treatment group will be followed for one year after implantation. The TE patch treated patients will be followed and continued to be assessed for SAE/AEs for 12 months post-treatment. For the first month after surgery, the treated patients will have a real-time Mobile Cardiac Outpatient Telemetry device that will instantly transmit an alert to a 24/7 monitoring service that can alert EMS in a life-threatening arrhythmia. During the 12-month follow-up, the TE patch treated patients with undergoing some measurements of efficacy: (1) CMR imaging to obtain data on changes in LV remodeling; (2) Quality of life questionnaire; (3) 6-minute walk test (6MWT) to obtain data on exercise capacity and potentially arrhythmia detection. We will also obtain blood for measurements of biomarkers during the trial (CRP, cTnI and NT-proBNP, Galectin (3). While these data will not be analyzed as part of the safety endpoints in the trial, they will provide information to use in planning future Phase II/III trials. Once the 12-month duration of the trial is complete, the TE patch treated patients will continue to be followed for 2 years by phone and/or email contact every 6-months to collect safety and feasibility data related to TE patch treatment.
To define the perioperative AEs and SAEs, 1-month data from the treatment group will be compared to AEs/SAEs from a chart review control group (CABG only) including patients from a population meeting the applicable inclusion/exclusion criteria, i.e., similar LV EF from the same study site(s). Control group information will be collected for up to 30 days post CABG. Specifically, to obtain data on the general event rates associated with CABG at the study site(s) in a similar time frame[25]. Published data may also be used to compare treatment and control (CABG only) groups.
Inclusion/exclusion criteria
The following study inclusion/exclusion criteria have been selected to optimize the ability of the patient to recover from surgery, participate in protocol follow up activities, and in post-procedure cardiac rehabilitation activities that may be provided or recommended by the study site(s) (participation in cardiac rehabilitation is not required to participate in the study).
Rationale for inclusion/exclusion criteria
This patient population was selected because it contains patients in whom the heart is already exposed during CABG. Thus, study participants will not have to undergo a separate surgical procedure for treatment with the TE patch. The study design requires that CABG be completed via median sternotomy so that the surgeon has maximal exposure and access to the heart during TE patch placement, like the methods used in pre-clinical studies in rats and swine. The study design requires that CABG be elective and scheduled in advance (for the treatment group). This eliminates higher risk, emergent procedures, and allows time to obtain baseline measurements for each participating patient. It is not expected that TE patch placement during CABG will significantly affect surgical or cardiopulmonary bypass time. In pre-clinical studies in swine, the TE patch was placed on swine hearts in less than 40 minutes and has not resulted in any surgical complications. Thus, the additional surgical risk to the TE patch treated patients is expected to be low in this patient population.
The inclusion criteria are essentially standard for a study in patients undergoing elective CABG, except that enrolled patients will have decreased LV function, i.e., EFs between 20% and 45%. While heart failure with reduced ejection fraction (HFrEF) is classically defined as EF < 40%, in our discussions with heart failure cardiologists and cardiac surgeons, we chose to enroll patients with EFs up to 45% to expand the potential patient population in the Phase I trial. As noted above, this is a proposed inclusion criterion that has yet to be approved by the FDA. In pre-clinical studies in rats and swine with MIs, the animals had decreases in LV function with maladaptive LV remodeling, which was partially reversed and with TE patch application. The exclusion criteria have been chosen to mitigate patient surgical and recovery risk. Out of an abundance of caution, patients with a recent history of sustained ventricular tachycardia have been excluded from the study. We have not observed sustained ventricular tachycardia in our animal models, nor do we expect patients to experience sustained ventricular tachycardia due to TE patch treatment. Patients in whom high-quality CMR images cannot be obtained at baseline (e.g., due to the patient having an ICD in place) will be excluded because we want to ensure that we obtain high-quality CMR images for feasibility data (volumes, scar size, etc.). Patients with autoimmune disease or patients on immunosuppression will be excluded to minimize the chance that a patient will become ill during the study because of having a compromised immune system. Further, this reduces potential concerns of the effect of immunosuppression on the implanted cells. Importantly, in our pre-clinical studies completed to date, data to support safety and efficacy were demonstrated in immune-competent animals receiving no immune suppression[16,18].
Justification of proposed endpoints in clinical trial
The primary endpoints will be related to the safety of TE patch. There are known potential complications from undergoing CABG alone, so for this reason, we will have a control group of patients undergoing CABG at the same site(s) with the same inclusion/exclusion criteria but not receiving a TE patch. In addition, to obtain data on AEs/SAEs from the surgical procedure, we will collect data on potential AEs/SAEs potentially directly related to TE patch such as pericarditis, edema around the TE patch, sustained ventricular tachycardia and unanticipated adverse immune responses causing unanticipated hospitalization, disability, or permanent damage. The issue of finding arrhythmias such as ventricular tachycardia in the TE patch treatment group is important because previous studies with injected skeletal myoblasts and embryonic stem cells resulted in the induction of ventricular arrhythmias[2,12]. Although a Phase I Clinical Trial is a safety trial, we have decided to include some potential efficacy endpoints. We realize that with this small sample size of 12 patients in the treatment group, we do not have adequate power to perform a statistical analysis of these endpoints, but preliminary data on these endpoints will help inform us in preparation for a Phase II/III trial.
Left ventricular remodeling and function, defined by CMR imaging
The foundation for using changes in LV remodeling, volumes, and mass/volume ratio, as efficacy endpoints date back to work documenting the benefits of angiotensin converting enzyme (ACE) inhibitors in rats with HFrEF published in 1985 in Circulation Research by Janice Pfeffer, Mark Pfeffer and Eugene Braunwald[26]. While Dr. Pfeffer and her colleagues did not measure EF or LV volumes in-vivo, they did obtain passive ex-vivo pressure/volume (P/V) relationships in rats with HFrEF after left coronary artery ligation and treatment with captopril. Their data showed that captopril put the left ventricle on a new P/V relationship that was shifted leftward toward the pressure axis and reduced operating LV end-diastolic volume. These investigators went on to show their rat data were relevant to patients, i.e., defining the beneficial effects of captopril in patients with anterior wall myocardial infarctions. These data were the basis for generating the hypotheses in the SOLVD and SAVE clinical trials, which showed that ACE inhibitors resulted in a survival benefit in patients with heart failure[27,28]. As such, ACE inhibitors and angiotensin receptor blockers have been part of the standard of care for patients as Guide Directed Medical Therapy in HFrEF for decades. One of the co-authors of these studies (MAP) commented: “At that time, we were studying the effects of progressive enlargement, and indeed the early use of captopril was designed to attenuate that time-dependent process. Work with cell-based therapy is now directed at reversing rather than preventing that process.” It is important to note that while the CMR volume data will allow us to calculate EF, the weaknesses of using EF as an index of LV contractile function have been well documented[29]. For this reason, we will measure Global Longitudinal Strain both by echocardiography and CMR.
Improving the relationship between LV mass relative to volume is important because LV volume increases relative to mass are associated with heightened wall stress during the entire cardiac cycle creating a stimulus for further adverse LV remodeling and a target for cell-based therapy. A patient with HFrEF can have a 3X increase in LV volume and an increase in wall thickness by 25%-75%, thereby doubling or tripling LV mass, predicting a poor prognosis[30]. Increasing LV mass is associated with increased extravascular compressive forces that may compromise subendocardial blood flow resulting in ischemia and increased arrhythmogenesis. Small changes in this ratio have long-term consequences. Lastly, changes in mass and volume alter LV shape, which is another aspect that adversely influences outcomes[31]. This supports our proposal that LV remodeling should be used to define outcomes in clinical trials of cell-based therapy.
Six-minute walk test
6MWT assesses distance walked over 6 minutes as a sub-maximal test of aerobic capacity/endurance. We will use the 6MWT to provide information on the change in the activity of our patients. This is important because the FDA supports endpoint measures that are directed at patient well-being, as opposed to just quantitative numbers such as EF or LV volume. We realize that patients are undergoing concomitant CABG, which will confound this evaluation.
Quality of life questionnaire
The quality-of-life questionnaire is also directed at patient well-being and suffers from the same issue as the 6MWT, i.e., it will be cofounded by the patient’s CABG. Nevertheless, we will collect this information hopefully to provide some insight into how the patients are doing.
IMPORTANT CAVEATS USING ALLOGENEIC HIPSC-CMS SEEDED ON TE PATCH
Mechanism(s) of action of bioengineered patch
While the mechanism(s) of action of hiPSC-CMs has not been clearly defined, most investigators believe that paracrine factors play an important role. Those specific factors are probably unique to the cells and/or combinations of cells being used. Our laboratory has shown a specific molecular profile consisting of upregulated endothelial, fibroblast, and cardiomyocyte markers in the graft in-vitro, as well as multiple growth factors and cytokines released from the graft itself after implantation in the immune component rats not receiving immune suppression[16,18]. Similar data have been reported with mesenchymal precursor cells that do not persist but generate soluble factors such as SDF-1, VEGF, and ANG-1[3]. An important component of the mechanism(s) of action of TE patch is the site of application. The fact that the TE patch is applied to the epicardial surface of the heart is important because of the epicardium that has been shown to be a “hub” for cardiac regeneration[32], as well as containing a common progenitor pool for the epicardium and myocardium[33,34].
No immune suppression for patients in the clinical trial
“We are not proposing to give patients any immune suppression in our clinical trial; in our pre-clinical studies, we implanted human cells in immune-competent animals, without immune suppression, and we achieved a therapeutic benefit while maintaining safety[16,18]. In the ongoing cell-sheet study with allogeneic hiPSC-CMs in Japan and in a clinical trial of injected allogeneic hiPSC-CMs in China, the patients are receiving short-term immune suppression (NCT04696328; NCT03763136). The use of immunosuppression is intended to promote the long-term persistence of the cells as an integrative approach. We are proposing a non-integrative strategy, where the cells will not integrate into the host tissue; this is similar to work done with MSCs[3,16,18]. The loss of cells over a short time and application to the epicardial surface of the heart may result in fewer ventricular arrhythmias in contrast to the experience with injected skeletal myoblasts and embryonic stem cells into the myocardium[2,12]. Recent data on immune responses to cell-based therapy has been shown that an immune response may be necessary for regenerating or rejuvenating the heart one week after an acute MI[35-38]. These same authors showed that mononuclear cells injected into the heart given 8 weeks after an MI failed to rejuvenate the scarred rodent heart[39]. These authors did conclude that since they only examined mononuclear cells, “future studies are warranted using other cell-based modalities”. This supports our work with hiPSC-CMs. The immune responses in cell-based therapy need more studies. For example, there are data that immune macrophage activity enhances native cardiomyocyte survival and contributes to neovascularization[3,40].
Our clinical trial will maintain patients on their standard therapy for CHF. This has been a standard requirement from the FDA for any additive therapy for such a severe disease as CHF. To date, this has not been a problem in any of the previous trials for cell-based therapy.
CONCLUSIONS
This perspective summarizes the development of a TE cell-based therapy to treat LV dysfunction and CHF, starting with an acellular collagen patch progressing to an allogeneic TE patch embedded with human neonatal fibroblasts and seeded with hiPSC-CMs. We outline the progress we have made in our pre-clinical studies starting with rats subjected to cryoinjury, to rats undergoing left coronary artery ligation, and then to the Yucatan mini swine model of 90 min ischemia/reperfusion of the LAD. The data obtained in these studies are the basis of a proposed Phase I clinical trial. The plan is to implant a TE patch onto the epicardial surface of patients’ hearts undergoing elective CABG with EFs ≥ 20% and ≤ 45%, with the primary endpoints being AEs/SAEs associated with placing the TE patch on the heart. While Phase I trials are primarily safety trials, the plan is to obtain some potential efficacy endpoints to help design future Phase II/III clinical trials. The justification for including these endpoints emphasizes changes in LV remodeling that occurred in the pre-clinical animal models as well as including endpoints that focus on patient well-being.
While the process of developing a TE patch has taken us years, we have benefitted from discoveries made by other investigators along the way, starting with the creation of pluripotent stem cells, differentiation into cardiomyocytes and production of high-quality hiPSC-CMs in unlimited quantities. Our work in cellular engineering resulted in the development of a bioengineered patch that is the foundation for co-culturing or seeding both human neonatal fibroblasts and cardiomyocytes onto the patch. The fibroblasts secrete an extracellular matrix that forms a functioning syncytium where the cardiomyocytes and fibroblasts can survive and secrete paracrine factors that are thought to be part of the mechanism of action resulting in physiologic benefit in pre-clinical studies. While these data are no guarantee that this TE patch will improve cardiac function in patients with LV dysfunction, we have outlined our pathway to get to this point.
DECLARATION
AcknowledgementsA special thank you to Mary Kaye Pierce RN, NP; Maribeth Stansifer, BS, Talal Moukabary, MD and Mark Borgstrom, PhD their technical assistance. We sincerely thank Drs. Taben Hale, Marc Alan Pfeffer, and Massimo Mangiola for their review and suggestions.
Authors’ contributionsCarried out the experiments, testing of the cardiac patch and are responsible for the initial draft of the manuscript: Goldman S, Lancaster JJ, Koevary JW
Reviewed the data, provided advice on the clinical trial design and reviewed/edited the manuscript: Traverse JH, Zile MR, Juneman E, Greenberg B, Kelly RF
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the Arizona Department of Health Services; Arizona Biomedical Research Centre Investigator Grant # ADHS16-162516, the NHLBI PACT Program for Cell Development PCT0009-2, the WARMER Research Foundation, the Sarver Heart Center, and the University of Arizona.
Conflicts of interestThe work outlined in this perspective were the basis of forming a commercial entity, Avery Therapeutics. Drs. Goldman, Koevary, and Lancaster have disclosed a financial interest in Avery Therapeutics, Inc. to the University of Arizona. In addition, the University of Arizona has a financial interest in Avery Therapeutics, Inc. These interests have been reviewed and are being managed by the University of Arizona in accordance with its policies on outside interests.
Ethical approval and consent to participateAll animal work was performed under oversight of the University of Arizona and/or Southern Arizona Veteran’s Administration Health Care System Institutional Animal Care and Use Committee (IACUC). The IACUC oversees the University of Arizona’s animal care and use program and is responsible for reviewing and approving all activities utilizing vertebrate animals for research, teaching and testing, ensuring compliance with federal animal welfare regulations, inspecting animal facilities and investigator laboratories, investigating animal concerns, and overseeing training and educational programs. PHS Animal Welfare Assurance Number: D16-00159 (A-3248-01), Effective August 8, 2019, Expires August 31, 2023. USDA Animal Research Facility Registration Number: 86-R-0003, Expires August 24, 2022. AAALACi Number (accredited since 1969): 000163, Continuing Full Accreditation (effective March 4, 2020).
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Savarese G, Lund LH. Division of Cardiology. Global public health burden of heart failure. Card Fail Rev 2017;3:7.
2. Liu YW, Chen B, Yang X, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 2018;36:597-605.
3. Borow KM, Yaroshinsky A, Greenberg B, Perin EC. Phase 3 DREAM-HF trial of mesenchymal precursor cells in chronic heart failure. Circ Res 2019;125:265-81.
4. Sun X, Wu J, Qiang B, et al. Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci Transl Med 2020;12:eaax2992.
5. Bolli R, Mitrani RD, Hare JM, et al. Cardiovascular Cell Therapy Research Network (CCTRN). A phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail 2021;23:661-74.
6. Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther 2005;105:151-63.
7. Thai HM, Juneman E, Lancaster J, et al. Implantation of a three-dimensional fibroblast matrix improves left ventricular function and blood flow after acute myocardial infarction. Cell Transplant 2009;18:283-95.
8. Lancaster J, Juneman E, Hagerty T, et al. Viable fibroblast matrix patch induces angiogenesis and increases myocardial blood flow in heart failure after myocardial infarction. Tissue Eng Part A 2010;16:3065-73.
9. Lancaster JJ, Juneman E, Arnce SA, et al. An electrically coupled tissue-engineered cardiomyocyte scaffold improves cardiac function in rats with chronic heart failure. J Heart Lung Transplant 2014;33:438-45.
10. Gao L, Kupfer ME, Jung JP, et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ Res 2017;120:1318-25.
11. Wnorowski A, Wu JC. 3-dimensionally printed, native-like scaffolds for myocardial tissue engineering. Circ Res 2017;120:1224-6.
12. Menasché P, Vanneaux V, Hagège A, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol 2018;71:429-38.
13. Kawamura M, Miyagawa S, Miki K, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012;126:S29-37.
14. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76.
15. Gaballa MA, Sunkomat JN, Thai H, Morkin E, Ewy G, Goldman S. Grafting an acellular 3-dimensional collagen scaffold onto a non-transmural infarcted myocardium induces neo-angiogenesis and reduces cardiac remodeling. J Heart Lung Transplant 2006;25:946-54.
16. Lancaster JJ, Sanchez P, Repetti GG, et al. Human induced pluripotent stem cell-derived cardiomyocyte patch in rats with heart failure. Ann Thorac Surg 2019;108:1169-77.
17. Sanchez P, Lancaster JJ, Weigand K, Mohran SE, Goldman S, Juneman E. Doppler assessment of diastolic function reflect the severity of injury in rats with chronic Heart failure. J Card Fail 2017;23:753-61.
18. Chinyere IR, Bradley P, Uhlorn J, et al. Epicardially placed bioengineered cardiomyocyte xenograft in immune-competent rat model of heart failure. Stem Cells Int 2021;2021:9935679.
19. Lancaster JJ, Koevary JW, Chinyere IR, Daugherty SL, Fox KA, Goldman S. Surgical treatment for heart failure: cell-based therapy with engineered tissue. Vessel Plus 2019;3:34.
21. Rajagopalan P, Shen CJ, Berthiaume F, Tilles AW, Toner M, Yarmush ML. Polyelectrolyte nano-scaffolds for the design of layered cellular architectures. Tissue Eng 2006;12:1553-63.
22. Ceccaldi C, Bushkalova R, Alfarano C, et al. Evaluation of polyelectrolyte complex-based scaffolds for mesenchymal stem cell therapy in cardiac ischemia treatment. Acta Biomater 2014;10:901-11.
23. Bushkalova R, Farno M, Tenailleau C, et al. Alginate-chitosan PEC scaffolds: a useful tool for soft tissues cell therapy. Int J Pharm 2019;571:118692.
24. Morris C, Ref JR, Acharya T Johnson KJ, et al. Free-breathing gradient recalled echo-based CMR in a swine heart failure model. Sci Rep 2022;12:3698.
25. Soukup CR, Schmidt CW, Chan-Tram C, Garberich RF, Sun BC, Traverse JH. Rate of Incomplete revascularization following coronary artery bypass grafting at a single institution between 2007 and 2017. Am J Cardiol 2021;144:33-6.
26. Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res 1985;57:84-95.
27. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
28. Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE investigators. N Engl J Med 1992;327:669-77.
29. Mann DL. Use of ejection fraction in heart failure: a tarnished gold standard? Journal of the American College of Cardiology - ACCEL Audio Journal 2019. Available from: https://www.acc.org/Education-and-Meetings/Products-and-Resources/ACCEL-Audio [Last accessed on 20 Apr 2022].
30. Kerkhof PL. Characterizing heart failure in the ventricular volume domain. Clin Med Insights Cardiol 2015;9:11-31.
31. Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 2011;58:1733-40.
33. Wei K, Serpooshan V, Hurtado C, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015;525:479-85.
34. Tyser RCV, Ibarra-Soria X, McDole K, et al. Characterization of a common progenitor pool of the epicardium and myocardium. Science 2021;371:eabb2986.
35. Vagnozzi RJ, Maillet M, Sargent MA, et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020;577:405-9.
36. Epstein JA, Rosenthal N, Feldman AM. Teasing the immune system to repair the heart. N Engl J Med 2020;382:1660-2.
37. Vagnozzi RJ, Kasam RK, Sargent MA, Molkentin JD. Cardiac cell therapy fails to rejuvenate the chronically scarred rodent heart. Circulation 2021;144:328-31.
38. Cardiovascular Research. Cardiovascular research discoveries. Available from: https://academic.oup.com/cardiovascres/pages/webinars [Last accessed on 20 Apr 2022] Eshenhagen T, Vagnozzi R. Cardiovascular Research Discoveries. Available from: https://www.youtube.com/watch?v=yEEwx5xIqHs [Last accessed on 20 Apr 2022].
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Goldman S, Traverse JH, Zile MR, Juneman E, Greenberg B, Kelly RF, Koevary JW, Lancaster JJ. Perspective on the development of a bioengineered patch to treat heart failure: rationale and proposed design of phase I clinical trial. Vessel Plus 2022;6:54. http://dx.doi.org/10.20517/2574-1209.2021.149
AMA Style
Goldman S, Traverse JH, Zile MR, Juneman E, Greenberg B, Kelly RF, Koevary JW, Lancaster JJ. Perspective on the development of a bioengineered patch to treat heart failure: rationale and proposed design of phase I clinical trial. Vessel Plus. 2022; 6: 54. http://dx.doi.org/10.20517/2574-1209.2021.149
Chicago/Turabian Style
Goldman, Steven, Jay H. Traverse, Michael R. Zile, Elizabeth Juneman, Barry Greenberg, Rosemary F. Kelly, Jen Watson Koevary, Jordan J. Lancaster. 2022. "Perspective on the development of a bioengineered patch to treat heart failure: rationale and proposed design of phase I clinical trial" Vessel Plus. 6: 54. http://dx.doi.org/10.20517/2574-1209.2021.149
ACS Style
Goldman, S.; Traverse JH.; Zile MR.; Juneman E.; Greenberg B.; Kelly RF.; Koevary JW.; Lancaster JJ. Perspective on the development of a bioengineered patch to treat heart failure: rationale and proposed design of phase I clinical trial. Vessel Plus. 2022, 6, 54. http://dx.doi.org/10.20517/2574-1209.2021.149
About This Article
Copyright
Data & Comments
Data
 Cite This Article 18 clicks
Cite This Article 18 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.