Stem cell-based therapy for myopic maculopathy: a new concept
Abstract
Myopia has reached epidemic proportions in the world, especially in East Asia. Pathologic myopia is an extreme type of high myopia that can cause irreversible blindness. Myopic maculopathy is one of the characteristics of pathologic myopia. Nowadays, limited treatments can preserve the visual outcome of these patients. We review the current treatment in practice for myopic maculopathy. Furthermore, based on the current stem cell-based therapy used in degenerative ocular diseases, we discuss a new concept of stem cell therapy for myopic maculopathy.
Keywords
INTRODUCTION
Myopia is one of the most common ocular disorders in the world, especially in East Asia. High myopia is the condition that eyes have refractive error ≤ -6.00 diopter when ocular accommodation is relaxed[1]. Pathologic myopia is an extreme type of high myopia with characteristic fundus changes including posterior staphyloma and myopic maculopathy equal to or more serious than diffuse choroidal atrophy[2].
The global prevalence of high myopia was 4.0% before 2016[3], and that of pathologic myopia was 0.9%-3.1% before 2014[4]. The prevalence of high myopia presents an increasing trend in recent years. It is estimated that 9.8% of the global population will have high myopia by 2050[3]. An even more astonishing fact is that the prevalence of high myopia in children aged 6-19 years reached 73% in East Asia before 2019[5]. Visual impairment caused by pathologic myopia will also increase correspondingly. Myopic retinopathy is the second leading cause of blindness and low vision in Chinese adults[6,7]. Both genetic and environmental factors contribute to the development of myopia[8,9]. Since the outbreak of COVID-19 in December 2019, students have been confined to the house and attending class online. Students spent more time on near work and less on outdoor activities. Home confinement has resulted in a significant myopic shift in students aged 6-8 years[10,11]. As Bullimore et al.[12] stated, even with a one diopter increase in myopia, the prevalence of myopic maculopathy would increase by 67%.
Because many patients with high myopia face the risk of blindness, treatments are urgently required to cease visual loss and preserve the remaining visual function. This manuscript reviews myopic maculopathy with an emphasis on current treatment modalities and conceptualized stem cell-based therapy.
Introduction of myopic maculopathy
Myopic maculopathy, also named myopic macular degeneration and myopic retinopathy, is defined as the condition comprising diffuse or patchy macular atrophy with or without lacquer cracks, choroidal neovascularization (CNV), and Fuchs’ spot[1]. Myopic maculopathy is seen in 22.9% of high myopes aged 7-70 years[13]. The severity of myopic maculopathy increases with older age and longer axial length (AL)[13-15]. Myopic maculopathy is not common in young patients. In patients around 20 years, myopic maculopathy is present in 8.3%[16]. In patients younger than 40 years, it is present in 18.7%[13]. In patients aged 40-70 years, the prevalence of myopic maculopathy increases to 58.3%[13].
The meta-analysis for pathologic myopia (META-PM) classification proposed by an international panel of myopia researchers in 2015 is used in pathologic myopia classification[17]. META-PM classification is based on photography [Figure 1]. There are five categories of myopic maculopathy [Table 1]: no myopic retinal degenerative lesion (Category 0) [Figure 1A]; tessellated fundus (Category 1) [Figure 1B]; diffuse chorioretinal atrophy (Category 2) [Figure 1C]; patchy chorioretinal atrophy (Category 3) [Figure 1D]; and macular atrophy (Category 4) [Figure 1E]. The categories indicate progressed chorioretinal atrophy. Additionally, three plus signs for lacquer cracks, myopic CNV, and Fuchs’ spot can be applied to any category. The plus signs indicate three statuses of myopic CNV (mCNV). Lacquer cracks are the breaks of RPE [Figure 1F], Bruch’s membrane (BM), and choriocapillaris. They provide an entrance of CNV to grow into subretinal space [Figure 1G]. Fuchs’ spot is the scar of mCNV with pigmentation[17] [Figure 1H]. The plus signs are lesions that could directly or potentially impair the central visual acuity. Among these three plus signs, only mCNV requires clinical interventions.
Figure 1. The fundus photography of each category of META-PM classification. (A) Category 0: no myopic retinal degenerative lesion. (B) Category 1: tessellated fundus. Thinning of the retina results in increasing visibility of the deep choroidal vessels. (C) Category 2: diffuse chorioretinal atrophy. An ill-defined yellowish-white lesion can be seen in the posterior pole. (D) Category 3: patchy chorioretinal atrophy. Multiple grayish-white, well-defined atrophy can be seen. The fovea is spared. (E) Category 4: macular atrophy. Atrophy involves the fovea. (F) Lacquer cracks (indicated by white arrow) in the background of patchy chorioretinal atrophy. (G) Active myopic CNV with bleeding (indicated by white arrow) in the background of patchy chorioretinal atrophy. (H) Fuchs’ spot (indicated by white arrow) in the background of macular chorioretinal atrophy. META-PM: meta-analysis for pathologic myopia; CNV: choroidal neovascularization.
META-PM classification for myopic maculopathy[17]
| Category | Plus lesion |
| Category 0: No myopic atrophy | Lacquer cracks |
| Category 1: Tessellated fundus | Myopic CNV |
| Category 2: Diffuse chorioretinal atrophy | Fuchs’ spot |
| Category 3: Patchy chorioretinal atrophy | |
| Category 4: Macular atrophy |
This classification system provides ophthalmologists with a simplified and uniform system to categorize myopic maculopathy by severity. The characteristic alternations in each category are presented in Figure 2.
Figure 2. The characteristic alternations of each category based on META-PM classification. The thickness of retina, choroid, and sclera are decreasing along with a higher grade of myopic maculopathy. Tessellated fundus is characterized by hypoplasia of the RPE, reduced filling of the choriocapillaris, and increased pigmentation of the choroidal stroma. Diffuse chorioretinal atrophy is characterized by a marked reduction of all layers of choroidal vessels. In the diffuse chorioretinal atrophy stage, the outer retina and RPE are present even though the choroid is absent. Patchy chorioretinal atrophy is characterized by the absence of the outer retina layers, RPE, and all layers of the choroidal vessels. The inner retina directly touches the sclera. The Bruch’s membrane is no longer intact. The structural alternations of macular atrophy are the same as those of patchy chorioretinal atrophy. RPE: retinal pigment epithelium; BM: Bruch’s membrane.
Category 1: tessellated fundus
Tessellated fundus, or tigroid fundus, is the preliminary sign of myopia. It results from thinning of the retina and increasing visibility of the deep choroidal vessels[16]. The factors related to the cause of fundus tessellation include hypoplasia of the retinal pigment epithelium (RPE), reduced filling of the choriocapillaris, and increased pigmentation of the choroidal stroma[18]. Tessellated fundus is not associated with a decline in best-corrected visual acuity (BCVA)[17]. Tessellated fundus can progress to the next category in a matter of time. Progression was seen in 19% of tessellated fundus in one ten-year observation[14], and up to 40% high myopic patients with only tessellated fundus or no myopic retinal lesions progressed to myopic maculopathy in follow-ups of more than ten years[15,19].
Category 2: diffuse chorioretinal atrophy
Diffuse choroidal atrophy is the ill-defined yellowish-white lesion in the posterior pole[17]. Diffuse atrophy starts around the optic disc, extends to the macular, and eventually covers the entire posterior pole[20]. Compared with tessellated fundus, patients have thinner choroid and worse visual acuity[21]. A pronounced loss of choriocapillaris, medium, and large-size choroidal vessels can be seen at this stage[2]. The thickness of choroid and sclera decreased due to the elongation of AL, while the thickness of Bruch’s membrane stayed the same[22]. Because the outer retina and RPE remain even though the choroid is absent, the visual acuity of patients is relatively preserved[2].
Category 3: patchy chorioretinal atrophy
Patchy chorioretinal atrophy is defined as grayish-white, well-defined atrophy. In this stage, the outer retina layers, RPE, choriocapillaris, and medium- and large-sized choroidal vessels are lost, and the inner retina directly touches the sclera[23]. It is different from diffuse atrophy because the Bruch’s membrane is no longer intact in this stage[24]. Patchy atrophy can develop from lacquer cracks, progress from diffuse chorioretinal atrophy, and can also be seen along the border of the posterior staphyloma[19]. Patchy atrophy usually develops away from the foveal region and expands and merges with each other[19]. Although the central fovea is spared in this stage, deficiency of supply from the choroid still results in visual decrease[19].
Category 4: macular atrophy
Macular atrophy is the final stage of myopic maculopathy, with a well-defined, whitish chorioretinal atrophic lesion at the fovea. In both patchy atrophy and macular atrophy, the Bruch’s membrane defect is smaller than the RPE defect in most cases[23,25]. Macular atrophy can be sub-divided into CNV-related macular atrophy (atrophy develops around a regressed CNV) and patchy atrophy-related macular atrophy (one progressed from patchy atrophy). CNV-related macular atrophy almost always develops in CNV patients after a five-year follow-up, and the visual acuity gradually drops, accompanied by enlargement of macular atrophy[26]. CNV-related macular atrophy develops around the scarred CNV and enlarges concentrically[27]. Patchy atrophy-related macular atrophy is more likely a fusion of multiple patchy atrophies[15]. Patients with older age, longer AL, and existing patchy atrophy and lacquer cracks are prone to develop macular atrophy[19]. Patients with a more severe category of myopic maculopathy are more inclined to experience progression. In an 18-year follow-up, progression was also found in 63.7% of patients with diffuse choroidal atrophy, 97.4% of patients with patchy chorioretinal atrophy, and 100% of patients with macular atrophy[15].
Myopic choroidal neovascularization
Introduction of myopic choroidal neovascularization
Myopic CNV (mCNV) is a major cause of irreversible blindness in pathologic myopia, especially in patients < 50 years of age[28]. It causes sudden visual loss on the onset and progressive decline in the natural course[26]. The prevalence of mCNV is reported to be 5.2%-11.3%, and 15% of patients have bilateral CNV[4]. In patients with pre-existing mCNV, the incidence of mCNV will increase in the fellow eye, with a rate of 34.8% in patients with pre-existing mCNV and 6.1% in patients without pre-existing mCNV[29]. Myopic CNV can occur in any degree of myopia[30]. It is estimated that CNV develops in 3.7% and 20% of myopic patients with diffuse chorioretinal atrophy and patchy atrophy, respectively[29]. The final visual outcome is unfavorable even after the application of therapy in most cases[31].
There are three phases of the natural course of mCNV: the active phase, the scar phase, and the atrophic phase[17]. The active phase is determined by the presence of hemorrhage and serous retinal detachment. The scar phase, also called Fuchs’ spot, is a dry lesion with a grayish-white scar appearance and sometimes pigmented. When the mCNV progresses into the atrophic phase, the CNV lesion flattens, and chorioretinal atrophy develops around the CNV[32].
Myopic CNV is mostly located at the fovea[33], but it is occasionally located next to the myopic conus[34]. Different from other types of CNV, mCNV is usually small in size and accompanied by little subretinal fluid[35]. Bleeding from mCNV can be absorbed spontaneously in a range of 1-15 months[26,36]. Most of the mCNV remains stable or regressed[36]. Rebleeding occurs in 22% in ten-year follow-up[26].
Risk factors of myopic choroidal neovascularization
The factors associated with the visual outcome of mCNV are the age of onset, follow-up duration, CNV location, and CNV lesion size. Yoshida et al.[33] found that patients ≤ 40 years old at the onset of mCNV had a better visual prognosis than those > 40 years old three years after diagnosis. Kojima et al.[37] pointed out that the aging RPE cells might lead to less restriction on CNV growth, and then the incompetent RPE cells led to delayed regression of CNV and development of chorioretinal atrophy around the CNV. The visual outcome also depends on follow-up periods. In another study, Yoshida et al.[26] investigated the influence of older age. In this study, a group of mCNV patients with a mean age of 46.9 years old were followed up for more than ten years. In the first three years, more than half of them maintained the same visual acuity as the initial value (≥ 20/200). In the tenth year, the visual acuity of 96.3% of patients dropped to < 20/200. Meanwhile, Yoshida et al.[26] found chorioretinal atrophy developed in the majority of the eyes after 5-10 years. Therefore, they postulated that the development of CNV-related macular atrophy was responsible for visual loss. On the contrary, patients with younger age and smaller CNV located in the juxtafoveal region had a favorable visual acuity better than 20/40 in a 5-year follow-up[32,38].
Pathogenesis of myopic choroidal neovascularization
The precise pathogenesis of mCNV is still unclear. Mechanical stress, hemodynamic changes, inflammatory factor, and genetic factor have been postulated.
Progressive thinning of the sclera and ectasia of the posterior sclera are the features of pathologic myopia. Similar to the sclera, the choroid becomes thinner during the progression of myopia. The thinning of choroid results from the loss of choriocapillaris and stroma and a reduction in the number of large choroidal vessels[39]. Mechanical stretching of the sclera plays a role in the development of mCNV[40]. In patients with pathologic myopia, the atrophic choroid and sclera make it easy to visualize retrobulbar structures by swept-source optical coherence tomography (SS-OCT) or enhanced depth imaging optical coherence tomography (EDI-OCT). Scleral perforating vessels are linear uniform hyporeflective structures running through the sclera[41]. The choroid circulation receives blood supply from short posterior ciliary arteries (SPCAs). Scleral perforating vessels are originated from SPCAs, which penetrate the sclera in the peripapillary and macular regions[42,43]. The scleral perforating vessels were detected at the site of CNV and lacquer cracks in 70%-80% of mCNV patients[44-46]. The perforating vessels induce weakness of the local sclera. Meanwhile, the thin choroid in high myopia patients is not competent to buffer the mechanical stress caused by myopia development. In patients with the thin choroid, the force would concentrate in the area of scleral perforating vessels and cause the disruption of RPE and Bruch’s membrane. In this way, the mCNV grows through the fissure into the subretinal space and tries to fix the mechanical break[45].
Loss of choroidal vessels causes a choroidal circulatory disturbance, induces hypoxia in the RPE and glial cells, and triggers upregulation of vascular endothelial growth factor (VEGF) expression[47]. VEGF is a family of proteins including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor[48]. VEGF-A is the key regulator for angiogenesis[48]. The receptors of VEGF are VEGFR-1, VEGFR-2, and VEGFR-3. The former two are expressed predominantly on vascular endothelial cells, while VEGFR-3 is expressed on lymphatic endothelial cells[49]. VEGF interacts with VEGFR-1 and VEGFR-2 to initiate an intracellular cascade and induce proliferation and migration of vascular endothelial cells and inhibit apoptosis[50]. In addition to hypoxia-induced expression of VEGF, the mechanical stress posed on RPE induces expression and secretion of VEGF[51].
VEGF, as a common contributor in different types of CNV, can be detected in aqueous humor and vitreous body. The physiologic expression of VEGF levels is lower in the eyes of highly myopic patients than those of non-myopia patients[47]. The VEGF level elevates in myopic patients with CNV compared to those without CNV[47]. When compared with other types of CNV, the VEGF level in the aqueous humor of mCNV patients was lower[52,53]. Several possible explanations for this have been proposed. Firstly, dysfunction of RPE cells causes less expression of VEGF. VEGF, which is expressed by differentiated RPE cells, is essential in the maintenance of choriocapillaris[54,55]. In high myopia, the deteriorated function of RPE cells causes a reduction of VEGF production and corresponding atrophy of choriocapillaris[56]. Secondly, the large vitreous cavity of myopic eyes could dilute VEGF concentration. In highly myopic patients, whether with CNV or not, the VEGF concentration is negatively correlated with AL[47,57]. Researchers have argued that the limited amount of VEGF localized to mCNV is not sufficient to distribute into the anterior chamber. Thirdly, the decrease of VEGF production is due to retinal thinning in myopic eyes. Sawada et al.[52] proposed that retinal thinning in myopia patients causes a relative increase in choroidal perfusion. Consequently, retinal hypoxia would be improved, and VEGF production would decrease, correspondingly.
In addition to hypoxia-induced expression of VEGF, the aqueous cytokines indicate that inflammation has a role in the development of mCNV[58]. In the study by Yamamoto et al.[58], the levels of interleukin-8 (IL-8) and VEGF were significantly higher in eyes with mCNV. IL-8 is a proangiogenic and inflammatory chemokine that can upregulate VEGF expression in ischemic circumstances[59]. Beyond that, the interleukin-10 (IL-10) and monocyte chemoattractant protein-1 (MCP-1) levels were higher in myopic eyes than in normal eyes in this study. Furthermore, IL-8 and MCP-1 were related to the severity of myopic maculopathy. MCP-1 is a chemokine that regulates monocyte chemotaxis and lymphocyte differentiation in inflammatory diseases[60]. Thus, it is suggested that inflammation is involved in the progression of myopic maculopathy and the etiology of mCNV.
Genetic factors also play a part in the development of mCNV. Leveziel et al.[61] compared mCNV cases with high myopia cases and evaluated 15 age-related macular degeneration genetic variants which could also be involved in mCNV development. They found that the rs10033900 single nucleotide polymorphism (SNP) located in CFI gene was significantly associated with mCNV in Caucasians. CFI encodes complement factor I (CFI), which is a complement regulatory protein in the classical, alternative, and mannose-binding lectin pathways of the complement system[62]. CFI is expressed by hepatocytes, macrophages, lymphocytes, endothelial cells, and fibroblasts[63]. CFI works as a protease to prevent positive amplification of complement cascade by cleaving C3b and C4b[64]. Leveziel hypothesized that the specific genetic effects related to the inflammatory pathway would increase the risk of mCNV.
In another large case-control study conducted by Miyake et al.[65] in Japan, rs10033900 was not found in mCNV patients, but rs12603825 in PEDF gene is possibly associated with mCNV patients whose AL is ≥ 29 mm. Pigment epithelium-derived factor (PEDF) is a member of the serine protease inhibitor superfamily[66]. It was first identified as a potent inducer of neuronal differentiation in cultured retinoblastoma cells[67]. In ocular tissue, PEDF is secreted by photoreceptors and RPE cells and released into the interphotoreceptor matrix between RPE and photoreceptors as well as in the inner retina[68,69]. It is also expressed in cornea and ciliary epithelium[70] and can be detected in the vitreous body and aqueous humor. PEDF is a multifunctional protein with antiangiogenic[71], neurotrophic[72], and antioxidant[73] properties in ocular tissue. PEDF exerts its antiangiogenic effect by inducing apoptosis of endothelial cells and interfering with VEGF-induced angiogenesis[74]. In a laser-induced CNV rat model, decreased expression of PEDF was observed at the injury sites, suggesting permission on the formation of CNV[75]. In addition, PEDF protects RPE monolayer and pericytes from oxidant stress[76,77], as well as protects photoreceptors by suppression of apoptotic and inflammatory pathways[78]. According to Costagliola et al.[53], after anti-VEGF therapy, the level of VEGF decreases, while the PEDF level increases in the aqueous humor of patients with mCNV. It is known that the development of CNV is an imbalance between angiogenic and antiangiogenic factors. Therefore, the SNP in the PEDF gene could be a biological marker associated with mCNV.
The ATN classification
Compared with the META-PM classification, the atrophy-traction-neovascularization (ATN) classification proposed by Ruiz-Medrano et al.[79] in 2019 takes tractional and neovascular components into consideration. ATN represents three key factors in myopic maculopathy: atrophy (A), traction (T), and neovascularization (N). The ATN classification is based on both fundus photography and OCT to assess myopic maculopathy. In 2021, Ruiz-Medrano et al.[80] updated the ATN system [Table 2]. Based on longer AL and worse BCVA, they defined severe pathologic myopia as eyes graded ≥ A3, ≥ T3, and/or ≥ N2, which indicate a significant myopic macular complication. The atrophic and neovascular classifications in ATN system are the same as META-PM system. Both systems have five classifications on the aspect of atrophy and three statuses of mCNV. The ATN system includes the tractional component, which is closely related to visual acuity[79].
Updated ATN classification for myopic maculopathy[80]
| Atrophy (A) | Traction (T) | Neovascularization (N) |
| A0: No myopic atrophy | T0: No macular schisis | N0: No myopic CNV |
| A1: Tessellated fundus | T1: Inner or outer foveoschisis or lamellar macular hole | N1: Macular lacquer cracks |
| A2: Diffuse chorioretinal atrophy | T2: Inner and outer foveoschisis | N2a: Active CNV |
| A3: Patchy chorioretinal atrophy | T3: Foveal detachment | N2b: Fuchs’ spot |
| A4: Complete macular atrophy | T4: Full-thickness macular hole | |
| T5: Macular hole and retinal detachment |
CURRENT INTERVENTIONS TO MYOPIC MACULOPATHY
Currently, no available treatment has been found for myopic maculopathy except for mCNV. Management of mCNV is the same as other types of CNV by laser photocoagulation, verteporfin photodynamic therapy, and anti-VEGF therapy. Approaches have been proposed to retard myopic progression including pharmacologic, surgical, and optical intervention.
Non-surgical interventions
Pharmacologic intervention (e.g., low concentration atropine) and optical intervention (e.g., orthokeratology) are used to slow down myopic progression before the development of myopic maculopathy.
Low concentration atropine
The Atropine in the Treatment of Myopia (ATOM1) study in 2006 suggested that 1% of atropine can slow down the progression of childhood myopia[81]. This study recruited 400 children (6-12 years old) with low to moderate myopia (spherical equivalent of -1.00 to -6.00 D and astigmatism of ≤ -1.5 D). Participants were randomly assigned to the treatment group (1% atropine) or control group (vehicle eye drops). Only one eye of the participants received treatment. After a two-year follow-up, the progression of spherical equivalent (SE) value was 0.28 ± 0.92 D in the atropine group and 1.20 ± 0.69 D in the control group. The AL remained unchanged in the atropine group, while the AL elongated 0.38 ± 0.38 mm in the control group. Overall, 77% of the atropine group demonstrated a reduction in progression of myopia compared with the control group. ATOM1 demonstrated the efficiency of 1% atropine on myopia control.
The prominent side effect of 1% atropine resulted from mydriasis and cycloplegia. Therefore, lower doses of atropine were used in the ATOM2 study. In the ATOM2 study (phase 1)[82], 0.5%, 0.1%, and 0.01% atropine were used in 400 children (6-12 years old) with myopia of at least -2.00 D and astigmatism of ≤ -1.50 D. Different concentrations of atropine were used in each group bilaterally for two years. The increase in SE value was 0.30 ± 0.60, 0.38 ± 0.60, and 0.49 ± 0.63 D in the 0.5%, 0.1%, and 0.01% atropine groups, respectively. The increase in AL was 0.27 ± 0.25, 0.28 ± 0.28, and 0.41 ± 0.32 mm, respectively, in the groups. A small clinical difference was found among the three treatment arms. The results of ATOM2 suggested that 0.5%, 0.1%, and 0.01% atropine were effective in reducing myopia progression, and higher doses achieved greater effect. Then, the 400 children underwent a one-year washout phase (phase 2)[83]. In this study, different degrees of myopic rebound after cessation of atropine were found in these three groups. The SE value increased 0.87 ± 0.52, 0.68 ± 0.45, and 0.28 ± 0.33 D in the 0.5%, 0.1%, and 0.01% atropine groups, respectively. The Al increased by 0.35 ± 0.20, 0.33 ± 0.18, and 0.19 ± 0.13 mm, respectively, in the groups. The rebound was greater in eyes in the 0.5% and 0.1% groups. During the entire 36 months of phase 1 and 2 ATOM2 studies, the overall increase of SE value was 1.15 ± 0.81 D in the 0.5% atropine group, 1.04 ± 0.83 D in the 0.1% atropine group, and 0.72 ± 0.72 D in the 0.01% group. The results suggest an inverse correlation between doses and myopic increase.
Since the rapid progression of myopia was found in children after cessation of atropine, phase 3 of ATOM2 was conducted[84]. Children in previous studies with a myopic progression of more than 0.5 D were recruited. Overall, 24% of children in the 0.01% group required the phase 3 treatment, while 59% in the 0.1% group and 68% in the 0.5% group needed further treatment. In total, 192 children restarted on 0.01% atropine for 24 months. In the entire five-year follow-up, the overall progression of myopia was slowest in the 0.01% group (SE 1.38 ± 0.98 D; AL 0.75 ± 0.48 mm), followed by the 0.1% (SE 1.83 ± 1.16 D; 0.85 ± 0.53 mm) and 0.5% groups (SE 1.98 ± 1.10 D; 0.87 ± 0.49 mm). Therefore, these studies suggested that 0.01% atropine is an effective treatment in myopic children, especially in those with rapid progression.
Orthokeratology
Orthokeratology (Ortho-K) is a process of reversibly reshaping the cornea by utilizing contact lenses overnight. The efficacy of Ortho-K on myopic control has been proved in clinical trials which recruited children (6-16 years old) with low to moderate myopia. Compared with the single-vision spectacles group, the axial elongation of the Ortho-K group was reduced by 36%-63% in the ROMIO study (the Retardation of myopia in Orthokeratology)[85], HM-PRO study (the High Myopia-Partial Reduction Ortho-k study)[86], and other clinical trials[87-89]. According to the existing studies, Ortho-K showed benefits to children with low to moderate myopia.
Interventions to myopic CNV
Anti-VEGF therapy
As mentioned above, the angiogenic stimulant VEGF is involved in the pathogenesis of mCNV. Therefore, VEGF is the target to treat choroidal angiogenesis. Nowadays, the widely used anti-VEGF drugs include ranibizumab, aflibercept, and conbercept. These drugs are divided into two groups based on their molecular structure. Ranibizumab is a recombinant, humanized, monoclonal antibody. It is an antigen-binding fragment (Fab) that can neutralize all isoforms of VEGF-A[90]. Aflibercept and conbercept are fusion proteins made of key domains of VEGFR1 and VEGFR2 fused with a portion of human antibody[91]. They act as a decoy receptor for VEGF and prevent VEGF from activating the real VEGFR[92].
Recently, anti-VEGF therapy has become the major treatment and provided good visual acuity benefits[93]. The safety and efficacy of these anti-VEGF agents have been proved in many studies. Similar visual gains and morphological improvements of mCNV patients were achieved by each of these anti-VEGF agents[94-96]. In the REPAIR study published in 2013[97], the efficacy of intravitreal 0.5 mg ranibizumab in mCNV was evaluated. Eyes with mCNV received ranibizumab at baseline and monthly injections as needed. Patients gained 13.8 letters at 12 months on average. The median number of injections was three. In the MYRROR study published in 2015[97], the efficacy of intravitreal aflibercept was evaluated. Patients were divided into the aflibercept and sham groups. Patients in the aflibercept group received one injection of 2 mg aflibercept at baseline and additional injections monthly when mCNV persisted or recurred. Primary efficacy was assessed at Week 24; patients in the aflibercept group gained 12.1 letters, while patients in the sham group lost two letters on average. Then, patients in the sham group received mandatory aflibercept injections for another 24 weeks with the same regimen. By Week 48, patients gained 13.5 and 3.9 letters in the aflibercept and sham/aflibercept groups, respectively. In the aflibercept group, the median number of injections was two during the first eight weeks, and no additional injections were applied during the remaining 40 weeks. In the sham/aflibercept group, the median number of injections was two during Weeks 25-36 and one during Weeks 37-48. Different from exudative age-related macular degeneration or diabetic macular edema, which requires frequent injection[98,99], the management of mCNV required a limited number of injections.
Severe adverse effects have been reported, including subretinal fibrosis[100], retinal detachment[100], and macular hole[101]. The common adverse events are conjunctival hemorrhage, transient increase in intraocular pressure, corneal punctate keratitis, and posterior capsule opacification[96]. Very few systemic complications associated with anti-VEGF therapy have been reported in clinical trials of mCNV patients. Due to favorable visual outcomes and safety profiles, anti-VEGF agents have become the standard treatment for mCNV[102].
Laser photocoagulation and photodynamic therapy
Laser photocoagulation and vPDT had been used to treat mCNV before the availability of anti-VEGF drugs in the past decades[103,104]. Laser photocoagulation is only employed in juxtafoveal CNV. The laser destroys the CNV and causes thermal damage in the outer retina, RPE, and choriocapillaris[105]. Successful laser photocoagulation could improve visual acuity because it prevents the CNV from extending to the fovea[106]. However, the long-term visual outcome is not favorable. Neovascular recurrence is one of the reasons for the deterioration of visual acuity. The incidence of recurrence ranges from 48.2% to 72%[106-108]. Furthermore, the laser-induced scar enlarged obviously in the first three months, accompanied by decreased visual acuity[109]. Expansion of the laser-induced scar is another cause of visual loss, and it has been regarded as a potential vision-threatening late complication[109].
PDT with verteporfin was a therapeutic modality to treat subfoveal CNV. Patients received an intravenous injection of a photosensitizer named verteporfin and application of a specific wavelength of laser light to the target lesion[110]. Verteporfin can bind to endogenous lipoproteins overexpressed on neovascular endothelial cells. After activation by laser, verteporfin leads to occlusion of the targeted CNV. Compared with laser photocoagulation, PDT induces selective occlusion of the CNV without injuring the surrounding neurosensory retina and RPE[111]. The efficacy and safety of PDT in mCNV have been approved[103,112,113], and the recurrence rate after treatment is around 50%[114,115]. In a meta-analysis, anti-VEGF therapy gained better vision in mCNV patients than photodynamic therapy (PDT) or laser photocoagulation[116]. In the RADIANCE study (Ranibizumab and PDT evaluation in myopic choroidal neovascularization) published in 2014[93], the efficacy and safety were compared between ranibizumab 0.5 mg intravitreal injection and verteporfin photodynamic therapy (vPDT). Patients with mCNV were randomly assigned to ranibizumab treatment guided by visual acuity stabilization criteria (Group 1), ranibizumab treatment guided by disease activity criteria (Group 2), and the vPDT group. Patients in the vPDT group could switch to ranibizumab three months after the initial vPDT. Ranibizumab achieved a rapid improvement in BCVA in Groups 1 and 2 during the first three months and a stable improvement up to the endpoint. The vPDT group improved lower BCVA compared to the ranibizumab group during the first three months. Then, 73% of participants with lower BCVA change in the vPDT group switched to the ranibizumab group. At 12-month follow-up, the mean BCVA improved 13.8 ± 11.42 letters in Group 1, 14.4 ± 10.2 letters in Group 2, and 9.3 letters in the vPDT group. Replicated injections were requested in the ranibizumab group. The median number of ranibizumab injections was four in Group 1 and two in Group 2. In addition to better visual outcomes in the ranibizumab groups, the RADIANCE study also demonstrated that an individualized regimen could achieve optimal visual outcomes.
Due to its advantages in terms of visual outcome and convenient intravitreal injection of drugs, anti-VEGF therapy has become the major treatment of mCNV[102].
Surgical interventions to myopic maculopathy
Posterior scleral reinforcement
Axial elongation contributes to myopic maculopathy. The thin sclera, especially the posterior pole of the globe, facilitates excessive ocular enlargement. Sclera is regarded as a prime target to stop the progression of myopia. Currently, reinforcement of the posterior sclera by different surgical methods is the only available way to managescleral ectasia.
Posterior scleral reinforcement (PSR) was first proposed by Shevelev[117] in 1930, modified by Borley and Snyder[118] in 1958, and then simplified by Thompson[119] in 1978. The surgical process has kept innovating in the following decades. The main procedure of PSR is placing a strip (sclera from donor’s eye, fascia lata strip[120] or duramater[121] from cadaver, or biomaterial patch) over the posterior pole to strengthen the posterior sclera and halt axial elongation. In most of the reports on PSR, the results are favorable with stable visual acuity as well as a lower increase in refractive error and AL[121-124]. According to Curtin’s grade of posterior staphyloma, the most common staphyloma developed on the nasal side of optic nerve[125]. Therefore, Curtin pointed out that PSR did not protect against staphyloma, which is not located in the macula[126]. In fact, the purpose of PSR is to preserve central visual acuity. Staphyloma located outside macula would not affect the visual outcome of PSR.
Despite mechanically strengthening the posterior sclera, PSR may halt axial elongation by ameliorating hypoxia of sclera. During myopia development, scleral hypoxia plays a pivotal role in sclera remodeling[127]. Choroidal ischemia is a possible cause of myopic maculopathy[128]. A thinner choroid is associated with faster AL growth[129]. Increasing choroidal vascular perfusion can improve oxygenation of the sclera and slow down myopia development in guinea pigs[130]. Zhang et al.[131] considered that the allogeneic scleral graft caused a secondary non-specific inflammatory reaction and increased the choroidal blood flow. Whether the improved choroidal circulation after surgery could prevent eye globe elongation still requires further investigation.
Currently, PSR is regarded as an effective way to limit the progression of pathological myopia and reduce the occurrence of myopic maculopathy, especially in patients with fast myopic progression. Studies on the treatment effect of PSR on pathological myopia are listed in Table 3.
Studies on the treatment effect of PSR on pathological myopia
| Authors | Age (year) | Follow-up duration | Control | Baseline AL (mm) | Endpoint AL (mm) | Elongation of AL in PSR group (mm) | Elongation of AL in control group (mm) | P |
| Xue et al.[124] | 7.5 | 10.8-43.2 m | Nonsurgical | 26.2 ± 1.33 | not mentioned | 0.75 ± 0.48 | 0.94 ± 0.44 | 0.0001 |
| Li et al.[217] | 41.03 ± 2.27 | 5 y | Nonsurgical | 29.49 ± 1.21 | 29.79 ± 1.26 | 0.30 ± 0.21 | 1.35 ± 0.56 | < 0.01 |
| Peng | 37.36 ± 16.22 | 3 y | Spectacles & contact lens | 29.42 ± 1.35 | 29.61 ± 1.61 | 0.23 ± 0.34 | 1.01 ± 0.33 | 0.0423 |
| Dong | 7.28 ± 3.69 | 3 y | Spectacles | 26.72 ± 1.28 | 27.00 ± 0.82 | 0.29 ± 0.33 | 0.82 ± 0.33 | < 0.0001 |
| Shen | 4.94 ± 0.77 | 3 y | Contact lens & patching | 26.78 ± 1.37 | 27.38 ± 1.30 | 0.60 | Not mentioned | 0.03 |
| Hu | 8.21 ± 3.86 | 1 y | Nonsurgical | 27.10 ± 1.02 | 27.23 ± 1.01 | 0.13 ± 0.17 | 0.71 ± 1.08 | < 0.05 |
| Chen | 6.50 ± 3.23 | 4.99 ± 1.3 y | Spectacles | 26.55 ± 1.60 | Not mentioned | 1.27 ± 0.54 | 2.05 ± 0.91 | < 0.001 |
Posterior pole buckle
Posterior pole buckle, or macular buckle, is similar to PSR. The main procedure of PSR is to reinforce the ectatic posterior pole, while that of posterior pole buckle is an application of stronger positive forces over the posterior pole to make an iatrogenic dome-shaped macula[132]. Due to scleral protrusion caused by silicone sponge, posterior pole buckle diminishes the high myopia[133]. The diminution degree is correlated with silicone sponge stretching and fixation. Posterior pole buckle is mostly applied in patients with macular membrane, macular schisis, and myopic macular hole with a retinal detachment to release the myopic macular traction[134]. It could be done alone or combined with pars plana vitrectomy[135,136].
The complications of both PSR and posterior pole buckles include lateral rectus weakness, intraocular pressure increase, diplopia, metamorphopsia, and choroidal effusion[134,137]. However, most complications are transient. Both PSR and posterior pole buckles are safe and effective with accurate placement of strip or buckle.
Scleral collagen cross-linking
Cross-linking was first used in keratoconus to increase the stiffness of cornea[138]. Then, in 2004, Wollensak[139] introduced scleral cross-linking for the treatment of high myopia. Cross-linking is the technique to enhance the tensile strength of collagen by the physical way (riboflavin–ultraviolet A[140]) or chemical agents (genipin[141] and glyceraldehyde[142]). Cross-linking strengthens the sclera by forming covalent bonds between collagen molecules[143] and reducing the enzymatic degradation by MMP1[144]. Until now, animal experiments including guinea pig[145], rat[146], and rhesus monkeys[147] have proved the efficiency and safety of scleral cross-linking, but no protocol has been brought to clinical use. Only one study conducted by Xue et al.[148] showed the genipin-crosslinked sclera strip used in PSR showed better strength and larger surgical effect size. Further clinical studies are required.
CURRENT STEM CELL-BASED THERAPIES FOR MACULOPATHY
Stem cell-based therapies have been mainly applied in degenerative corneal and retinal diseases. The following summarizes the stem cell-based clinical trials in maculopathy.
The stem cell ophthalmology treatment study
The Stem Cell Ophthalmology Treatment Study (SCOTS) is a series of clinical studies treating incurable optic and retinal diseases with autologous bone marrow-derived stem cells (BMSCs). The completed clinical trials include studies on Usher syndrome[149], dominant optic atrophy[150], Leber’s hereditary optic neuropathy[151], relapsing auto-immune optic neuropathy[152], and serpiginous choroidopathy[153]. In these studies, patients received subretinal or intra-optic nerve injection alone or a combination of retrobulbar, sub-Tenon’s, intravitreal, and intravenous injection. Meaningful visual acuity improvements were confirmed in these studies, and no adverse events were seen. Possible mechanisms in these studies were proposed: BMSCs released exosomes containing microRNA (miRNA) to regulate gene expression in inherited retinal degeneration, transferred mitochondria to rescue ganglion cells in mitochondrial disease, secreted growth factors to promote photoreceptors and neuron survival, and transdifferentiated into neurons or photoreceptors. Although there was a small number of patients enrolled, the favorable visual acuity and visual field improvement make autologous BMSC an option to treat these incurable diseases.
Stem cell-based therapy in macular degeneration
Age-related macular degeneration (AMD) is the most common cause of blindness in patients older than 55 years in developed countries[154]. There were about 196 million patients in 2020, and it was estimated that there will be 288 million in 2040[154]. The exact pathogenesis of AMD is obscure. Factors such as senescence, chronic inflammation, complement pathway, and lipid metabolism are proposed as implicated in the pathogenesis[155-158]. At the cellular level, AMD results from loss or dysfunction of RPE cells and secondary death of photoreceptors. According to the clinical manifestations, AMD is divided into dry (non-exudative or atrophic) and wet (exudative) types. Since the introduction of anti-VEGF treatments in 2004[159], the irreversible visual loss has been slowing down over the past decade[160]. However, the anti-angiogenesis treatment requires long-term repeat applications. Although these two types of AMD are different clinically, they both end up with a complete loss of photoreceptors, RPE, and choriocapillaris. Therefore, replacement therapy is the best way to restore vision.
Stargardt disease, also named Stargardt’s macular degeneration (STGD), is one of the most prevalent inherited juvenile macular dystrophies[161]. The early onset of STGD results in a visual loss in the working age population[162]. Three genes have been found responsible for three types of STGD. ABCA4 (1p22.1), ELOV4 (6q14.1), and PROM1 (4p15.32) are implicated in STGD1, STGD3, and STGD4, respectively. STGD1 is an autosomal recessive inherited disease caused by an ABCA4 gene mutation. This gene encodes the ATP-binding cassette transporters protein (ABCA4) expressed in the outer segment of photoreceptors. ABCA4 transports retinylidene-phosphatidylethanolamine (N-retinylidene-PE) from the inside of disk to the cytoplasmic side of the disk and avoids N-retinylidene-PE reacting with all-trans-retinal and forming di-retinoid-pyridinium-ethanolamine (A2E), which is toxic to photoreceptors and RPE[163]. STGD3 is an autosomal dominant inherited disease caused by a mutation in ELOVL4. This gene encodes ELOVL fatty acid elongase 4, which is involved in a catalyzing reaction of the long-chain fatty acids elongation cycle[164]. Mutated ELOVL4 causes the production of a truncated ELOVL4 protein lacking a motif for endoplasmic reticulum retention. Dysfunction of ELOVL4 protein results in decreased synthesis of long-chain (C28-C36) fatty acids. C28-C36 fatty acids are components of phosphatidylcholines which are distributed in photoreceptor outer segments[165], bind to rhodopsin[166], and regulate activities of photo-transduction proteins[167]. The truncated ELOVL4 protein causes deficiency of C32-C36 acyl phosphatidylcholines, alternation in photo-transduction proteins, and an increase in lipofuscin accumulation[168]. STGD4 is an autosomal dominant inherited disease caused by the mutation in PROM1 gene[169]. Prominin 1, encoded by PROM1, is located at the base of the photoreceptor outer segments and participates in disk membrane formation. Mutated PROM1 causes overgrown and misoriented outer segment disk membranes, which indicate defective disk morphogenesis[170]. Similar to AMD, complete loss of photoreceptors, RPE, and choriocapillaris is observed at the end stage of STGD.
The key functions of RPE cells are delivering nutrients and disposing waste for photoreceptors and phagocytosis of outer segments of photoreceptors[171]. The purpose of delivering RPE cells to the subretinal space is to rescue the remaining photoreceptors. The subretinal space is an immune-privileged environment suitable for cell-based therapy. Subretinal injection of cell suspension and the delivery of cell patches are new replacement therapy processes[172]. A clinical trial on stem cell transplantation for STGD and dry AMD was first conducted to prove the safety and tolerability of human embryonic stem cell-derived RPE (hESC-derived RPE) in 2012[173]. From then on, the innovation of cell transplantation focuses on sources of cells and approaches of delivery. Because polarized RPE cells array in a monolayer in physical conditions, researchers proposed that loading RPE cells on a bioengineered scaffold is an optimal method for delivery. A synthetic biocompatible membrane for RPE loading made from different materials has been innovated[174, 175], and some tested in clinical studies[176,177]. In 2017, iPSC-RPE cell sheets were first transplanted in one wet AMD patient[178]. Although the visual acuity did not improve, the implanted graft integrated well with the local neuroretina. Thus far, the safety of stem cell-based therapy has been proved, while the efficacy requires further investigation. A recent review comprehensively summarizes the current status of stem cell-based retinal regeneration[179].
CONCEPTUALIZED CELL THERAPY FOR MYOPIC MACULOPATHY
Cell therapy is substitutive therapy by regenerating dysfunctional tissues or cells with curative cells[180]. It is a novel method to treat diseases that were previously regarded as incurable. Here, we review the cells which are theoretically available for cell therapy for pathologic myopia.
Autologous iPSC-RPE
Hypoplasia and loss of RPE are characteristics during the progression of myopic maculopathy. As with other forms of macular degeneration, loss of RPE cells leads to apoptosis of photoreceptors in pathologic myopia. In patients with high myopic macular hole, the anatomical closure of the macular hole and visual improvements have been the main criteria for a successful surgery. There are reports that failed to improve visual acuity after the closure of macular hole[181]. Ophthalmologists are inclined to attribute the failure to the severity of pathologic myopia or disrupted photoreceptors[182,183]. However, the status of RPE was ignored in the past.
More attention needs to be paid to RPE. Fang et al.[184] studied 14 eyes with fovea-centered macular atrophy that developed after successful pars plana vitrectomy (PPV) for myopic traction maculopathy and macular hole retinal detachment. The postoperative visual acuity was even worse than the baseline. These eyes had diffused atrophy or extrafoveal patchy atrophy preoperatively and developed macular atrophy at a median time of 3.5 months after surgery. PPV-related macular atrophy is different from CNV- or patchy atrophy-related macular atrophy as it starts from the fovea. Eyes with macular hole retinal detachment are prone to develop PPV-related macular atrophy. This study emphasized the importance of RPE on the recovery of vision. When reviewing the postoperative outcomes of high myopic macular hole, AL > 30 mm and presence of posterior staphyloma are risk factors for unfavorable visual outcomes[185]. It is rational that a longer AL indicates severe chorioretinal atrophy and loss of RPE.
Therefore, transplantation of RPE cells seems a preferable way to improve the visual outcomes of high myopic patients by replacing atrophic RPE cells with healthy ones. The efficiency and safety of GMP-grade human iPSC-RPE were proved in a pre-clinical study conducted by Zhang et al.[172]. Here, the transplanted cells were injected into the subretinal space. However, a cell suspension is not practical to apply in myopic macular hole surgery. Scaffolds for RPE transplantation with excellent cytocompatibility and biocompatibility are required[174]. A cell patch is feasible to place under the macular hole during PPV surgery. The scaffolds act as the Bruch’s membrane for functional RPE reconstruction [Figure 3]. Compared with traditional surgery, combination with iPSC-RPE transplantation would reap greater benefits. One concern of RPE cell patch transplantation is the viability of the cell patch when there is a lack of choroidal perfusion from the severely atrophic choroid. Pang et al.[186], in 2015, identified that myopic eyes with an extremely thin choroid (≤ 20 μm) could still have BCVA ≥ 20/40. They proposed two explanations: (1) a fovea with extremely thin choroid would receive blood supply from larger patent choroidal vessels located eccentric to the subfoveal area; and (2) an atrophic retina with a thinner layer allows more oxygen to permeate from deep capillary plexus to outer retina. Therefore, we have reasons to believe that transplantation of RPE cell patches to eyes with myopic macular holes is feasible.
Figure 3. The schematic diagram of iPSC-RPE transplantation in pathologic myopia. Generate a cell patch by culturing iPSC-RPE cells with a cell scaffold. Then, transplant the cell patch into the subretinal space of pathologic myopic eyes. The excellent cytocompatibility and biocompatibility of the scaffold offer RPE cells a suitable circumstance for reconstruction. RPE: retinal pigment epithelium.
Fibroblasts/Myofibroblasts
The sclera, as the wall of eye globe, must have the strength to protect the delicate intraocular structures and some degree of elasticity to buffer fluctuations of intraocular pressure. The sclera is composed of collagens, proteoglycans, non-collagenous glycoproteins, and elastic fibers. In the human sclera, 90% of the components are collagen type I[187]. The collagens form the scaffolds, while proteoglycans and glycoproteins fill the interfibrillar space[188]. Collagen fibrils irregularly arrange and form layers. Fibroblasts intersperse between scleral collagen layers and synthesize sclera extracellular matrix components[189].
The thinning of sclera is a pivotal factor in the progression of myopia. In the histological study of enucleated human eye globes, the scleral volume increases with longer AL in the first two years of life and stays stable in the following years[190]. This finding indicates that scleral thinning due to axial elongation is contributed to the rearrangement of existing sclera tissue rather than synthesizing new sclera. In the posterior sclera of myopic eye, reductions in the number of fibroblasts and the collagen fibril diameter can be found[191]. In addition, unusual fibrils with a star shape on cross section increase in myopic sclera[191]. It is known that an increase of matrix metalloproteinase-2 (MMP-2) activity[192], a decrease of turnover rate of proteoglycan[193], and structural changes of collagen fibrils result in a weak and extensible sclera. The changed scleral biomechanics is thought to be responsible for the increasing AL and development of myopia.
Myofibroblasts are a population of scleral cells arising from fibroblasts. The transition is stimulated by mechanical stress, transforming growth factor-β (TGF-β), and cellular fibronectin[194]. Myofibroblasts are highly contractile cells that can express alpha-smooth muscle actin (α-SMA) to respond to scleral mechanical stress and limit expansion of the surrounding matrix[195]. Reduction in the level of TGF-β is a contributor to remodeling of ECM during the development of myopia[196]. TGF-β is important in the regulation of ECM turnover. A reduction of TGF-β would induce a decrease of α-SMA expression and cell-mediated contraction[196]. TGF-β not only induces myofibroblasts to synthesize α-SMA but also promotes the production of collagen type I[194]. Myofibroblasts contract stress fibers, which are attached to the surrounding ECM and cause local contraction of the matrix. Then, the myofibroblasts deposit ECM to stabilize the contraction[197].
Shinohara et al.[198] found that transplanting human dermal fibroblasts into sclera is an effective way to reduce axial elongation in form-deprivation myopia rats. The transplanted fibroblasts can synthesize new collagen fibrils with a bundle-like appearance and a stripe-like pattern. The newly synthesized collagen fibrils could reinforce the sclera and slow down the axial elongation of myopic eyes. An immunosuppressant was used in this study due to hetero-transplantation. It is feasible to use autologous iPSC-fibroblasts or autologous iPSC-myofibroblasts as a novel therapy to enhance the posterior sclera. Therefore, fibroblast or myofibroblast transplantation is a promising way to reduce the progression of myopia and prevent myopes from developing myopic maculopathy.
Scleral stem/Progenitor cells
Scleral stem/progenitor cells (SSPCs) have been isolated from murine sclera[199]. SSPCs express stem cell genes ABCG2, Six2, Pax6, and Notch1 and are positive for mesenchymal markers including Sca-1, CD90.2, CD44, CD105, and CD73. In addition, SSPCs can differentiate into adipogenic, chondrogenic, and neurogenic lineages[199]. However, related studies are rare, and investigations characterizing SSPCs are still required.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are stromal cells with the abilities of self-renewal and multilineage differentiation. MSCs can be found in various tissue in adults, including bone marrow, adipose tissue, peripheral blood, dental pulp, periosteum, and skeletal muscle[200-204]. In addition, fetal MSCs can be harvested from fetal tissues and extraembryonic tissues including placenta, amnion, amniotic fluid, and umbilical cord[205-207]. Numerous sources of MSCs have been found, but MSCs derived from bone marrow and adipose tissue are the main sources in current cell therapies. Due to its immunoregulatory capacity and immune privileges, MSCs have been applied in multi-system disease[208].
MSCs work both on innate immune response and adaptive immune response: MSCs induce division arrest anergy of immune cells[209]; prevent maturation of dendritic cells and activation of natural killer cells[210]; inhibit proliferation, differentiation, and chemotaxis of B cells; interfere with antibody production of B cells[211]; and induce T cell unresponsiveness by altering antigen-presenting cell maturation[212]. MSCs can migrate to sites of disease or injury and work as trophic mediators to release a broad range of bioactive molecules including growth factors, cytokines, and chemokines[213].
MSCs exert their therapeutic potential by paracrine effects instead of differentiating into tissue-specific cells[214]. A wide array of proangiogenic cytokines, such as VEGF, basic fibroblast growth factor (bFGF), and placental growth factor (PlGF), can be detected in the conditioned media of MSCs. Injection of the MSC media into murine ischemic limb markedly enhanced the limb’s perfusion[215]. Due to decreased choroidal perfusion and hypoxia of sclera in highly myopic patients, the conditioned media of MSCs offers a good option to modify the hypoxic circumstance of sclera. By injection of MSC conditioned media into the sub-Tenon’s space or suprachoroidal space, it can exert its angiogenic effect without the risk of tumorigenesis.
In addition to subretinal transplantation of iPSC-RPE, myofibroblasts, sclera stem cells, and MSCs are methods for posterior scleral reinforcement [Figure 4].
Figure 4. The cells which are theoretically available to cell therapy for reinforcement of sclera in pathologic myopia. Fibroblasts and myofibroblasts are scleral cells maintaining scleral biomechanics. Scleral stem cells might be the native stem cell in sclera. MSCs are stromal cells with the abilities of self-renewal and multilineage differentiation. MSCs exert their therapeutic effect by various paracrine growth factors. The media of MSCs also contains growth factors. Therefore, these cells and media can be made into products and delivered to the posterior sclera and fix the sclera. MSCs: Mesenchymal stem cells.
What challenges will we meet?
Although we can see the bright prospect of stem cell-based therapy for myopic maculopathy, there are several problems we need to consider.
When is the best therapeutic window?
In myopic patients with patchy or macular atrophy, subretinal transplantation of iPSC-RPE is a good choice to reduce the loss of photoreceptors secondary to RPE loss. However, the best therapeutic window is hard to elucidate. In patients with severe loss of photoreceptors, transplantation of sole RPE is of no help. Another situation is when patchy atrophy develops, sparing the fovea, and the patient maintains good visual acuity. In this case, a surgery with subretinal transplantation of iPSC-RPE poses a great risk to vision.
Difficulties in surgical technique
During the PPV surgery of subretinal transplantation of iPSC-RPE, experienced surgeons are required. Subretinal injections of RPE cell suspension or subretinal placement of RPE cell patch, especially in high myopia patients with thin retina, are elaborate procedures. The surgeons need to prevent transplanted cells from dispersing into the vitreous cavity, or they need to keep the cell patch in a specific position. In addition, specific surgical instruments are required. For example, a 25/41 G dual-bore cannula is used for subretinal bleb creation[216], and a special instrument is required for subretinal cell patch delivery.
CONCLUSION
Until now, the pathogenesis of pathologic myopia is still unclear. The only way to improve the prognosis of patients with pathologic myopia is to prevent the development of myopic maculopathy and restrain it in time. Anti-VEGF agents have partially preserved the vision of patients with mCNV, but the majority of patients with a higher level of myopic maculopathy face a high risk of visual loss. Eliminating blindness is the common goal of ophthalmologists and scientists. Cell-based therapy has opened a new era of treatment. Stem cell-based therapy has been regarded as the future of medicine and thrown light upon curing incurable diseases. Treatments by stem cell transplantation have made great progress in corneal and retinal degenerative diseases. It is hopeful that the atrophic structures in myopic eyes can be replaced by stem cells and renew the tissue. The preliminary work has shown a promising way in stem cell-based therapy of patients with myopic maculopathy. Therefore, it is possible to achieve the ambitious goal by stem cell-based therapy.
DECLARATIONS
AcknowledgmentsFigure 2 to Figure 4 in this article are created with BioRender.com. We thank all members of 502 team for the discussion.
Authors’ contributionsOriginal draft: Ma Y
Review & Editing: Li YP
Conceptualization, Funding acquisition, Supervision: Jin ZB
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work is partly supported by the National Key R&D Project (2017YFA0105300), Beijing Natural Science Foundation (Z200014) and National Natural Science Foundation of China (82125007).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Flitcroft DI, He M, Jonas JB, et al. IMI - Defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci 2019;60:M20-30.
2. Ohno-Matsui K, Wu PC, Yamashiro K, et al. IMI pathologic myopia. Invest Ophthalmol Vis Sci 2021;62:5.
3. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123:1036-42.
4. Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 2014;157:9-25.e12.
5. Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw SM. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol 2020;20:27.
6. Xu L, Wang Y, Li Y, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology 2006;113:1134.e1-11.
7. Liang YB, Friedman DS, Wong TY, et al. Handan Eye Study Group. Prevalence and causes of low vision and blindness in a rural chinese adult population: the Handan Eye Study. Ophthalmology 2008;115:1965-72.
8. Jin ZB, Wu J, Huang XF, et al. Trio-based exome sequencing arrests de novo mutations in early-onset high myopia. Proc Natl Acad Sci U S A 2017;114:4219-24.
9. Cai XB, Shen SR, Chen DF, Zhang Q, Jin ZB. An overview of myopia genetics. Exp Eye Res 2019;188:107778.
10. Wang J, Li Y, Musch DC, et al. Progression of myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol 2021;139:293-300.
11. Zhang X, Cheung SSL, Chan HN, et al. Myopia incidence and lifestyle changes among school children during the COVID-19 pandemic: a population-based prospective study. Br J Ophthalmol ;2021:bjophthalmol-2021.
12. Bullimore MA, Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci 2019;96:463-5.
13. Xiao O, Guo X, Wang D, et al. Distribution and severity of myopic maculopathy among highly myopic eyes. Invest Ophthalmol Vis Sci 2018;59:4880-5.
14. Yan YN, Wang YX, Yang Y, et al. Ten-year progression of myopic maculopathy: the beijing eye study 2001-2011. Ophthalmology 2018;125:1253-63.
15. Fang Y, Yokoi T, Nagaoka N, et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology 2018;125:863-77.
16. Koh V, Tan C, Tan PT, et al. Myopic Maculopathy and optic disc changes in highly myopic young asian eyes and impact on visual acuity. Am J Ophthalmol 2016;164:69-79.
17. Ohno-Matsui K, Kawasaki R, Jonas JB, et al. META-analysis for Pathologic Myopia (META-PM) Study Group. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol 2015;159:877-83.e7.
18. Yan YN, Wang YX, Xu L, Xu J, Wei WB, Jonas JB. Fundus tessellation: prevalence and associated factors: the beijing eye study 2011. Ophthalmology 2015;122:1873-80.
19. Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology 2010;117:1595-611, 1611.e1.
20. Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res 2016;52:156-87.
21. Wang NK, Lai CC, Chu HY, et al. Classification of early dry-type myopic maculopathy with macular choroidal thickness. Am J Ophthalmol 2012;153:669-77, 677.e1.
22. Jonas JB, Holbach L, Panda-Jonas S. Bruch’s membrane thickness in high myopia. Acta Ophthalmol 2014;92:e470-4.
23. Du R, Fang Y, Jonas JB, et al. Clinical features of patchy chorioretinal atrophy in pathologic myopia. Retina 2020;40:951-9.
24. Ohno-Matsui K, Jonas JB, Spaide RF. Macular bruch membrane holes in highly myopic patchy chorioretinal atrophy. Am J Ophthalmol 2016;166:22-8.
25. Jonas JB, Ohno-Matsui K, Spaide RF, Holbach L, Panda-Jonas S. Macular Bruch’s membrane defects and axial length: association with gamma zone and delta zone in peripapillary region. Invest Ophthalmol Vis Sci 2013;54:1295-302.
26. Yoshida T, Ohno-matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization. Ophthalmology 2003;110:1297-305.
27. Yokoi T, Ohno-Matsui K. Diagnosis and treatment of myopic maculopathy. Asia Pac J Ophthalmol (Phila) 2018;7:415-21.
28. Spaide RF. Choroidal neovascularization in younger patients. Curr Opin Ophthalmol 1999;10:177-81.
29. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol 2003;87:570-3.
30. Ikuno Y, Jo Y, Hamasaki T, Tano Y. Ocular risk factors for choroidal neovascularization in pathologic myopia. Invest Ophthalmol Vis Sci 2010;51:3721-5.
31. Farinha CL, Baltar AS, Nunes SG, et al. Progression of myopic maculopathy after treatment of choroidal neovascularization. Ophthalmologica 2014;231:211-20.
32. Hayashi K, Ohno-Matsui K, Yoshida T, et al. Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 2005;243:13-9.
33. Yoshida T, Ohno-matsui K, Ohtake Y, et al. Long-term visual prognosis of choroidal neovascularization in high myopia. Ophthalmology 2002;109:712-9.
34. Nagaoka N, Shimada N, Hayashi W, et al. Characteristics of periconus choroidal neovascularization in pathologic myopia. Am J Ophthalmol 2011;152:420-427.e1.
35. Lee DH, Kang HG, Lee SC, Kim M. Features of optical coherence tomography predictive of choroidal neovascularisation treatment response in pathological myopia in association with fluorescein angiography. Br J Ophthalmol 2018;102:238-42.
36. Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 1984;91:1573-81.
37. Kojima A, Ohno-Matsui K, Teramukai S, et al. Estimation of visual outcome without treatment in patients with subfoveal choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol 2006;244:1474-9.
39. Neelam K, Cheung CM, Ohno-Matsui K, Lai TY, Wong TY. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res 2012;31:495-525.
40. Mcbrien N. Role of the sclera in the development and pathological complications of myopia. Progress in Retinal and Eye Research 2003;22:307-38.
41. Ohno-Matsui K, Akiba M, Ishibashi T, Moriyama M. Observations of vascular structures within and posterior to sclera in eyes with pathologic myopia by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53:7290-8.
42. Moriyama M, Ohno-Matsui K, Futagami S, et al. Morphology and long-term changes of choroidal vascular structure in highly myopic eyes with and without posterior staphyloma. Ophthalmology 2007;114:1755-62.
43. Ishida T, Watanabe T, Yokoi T, Shinohara K, Ohno-Matsui K. Possible connection of short posterior ciliary arteries to choroidal neovascularisations in eyes with pathologic myopia. Br J Ophthalmol 2019;103:457-62.
44. Xie J, Chen Q, Yu J, et al. Morphologic features of myopic choroidal neovascularization in pathologic myopia on swept-source optical coherence tomography. Front Med (Lausanne) 2020;7:615902.
45. Giuffrè C, Querques L, Carnevali A, De Vitis LA, Bandello F, Querques G. Choroidal neovascularization and coincident perforating scleral vessels in pathologic myopia. Eur J Ophthalmol 2017;27:e39-45.
46. Querques G, Corvi F, Balaratnasingam C, et al. Lacquer cracks and perforating scleral vessels in pathologic myopia: a possible causal relationship. Am J Ophthalmol 2015;160:759-66.e2.
47. Wakabayashi T, Ikuno Y, Oshima Y, Hamasaki T, Nishida K. Aqueous concentrations of vascular endothelial growth factor in eyes with high myopia with and without choroidal neovascularization. J Ophthalmol 2013;2013:257381.
49. Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 2006;39:469-78.
50. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB j 1999;13:9-22.
51. Seko Y, Seko Y, Fujikura H, et al. Induction of vascular endothelial growth factor after application of mechanical stress to retinal pigment epithelium of the rat in vitro. Invest Ophthalmol Vis Sci 1999;40:3287-91.
52. Sawada O, Kawamura H, Kakinoki M, Sawada T, Ohji M. Vascular endothelial growth factor in the aqueous humour in eyes with myopic choroidal neovascularization. Acta Ophthalmol 2011;89:459-62.
53. Costagliola C, Semeraro F, dell'Omo R, et al. Effect of intravitreal ranibizumab injections on aqueous humour concentrations of vascular endothelial growth factor and pigment epithelium-derived factor in patients with myopic choroidal neovascularisation. Br J Ophthalmol 2015;99:1004-8.
54. Ohno-Matsui K, Morita I, Tombran-Tink J, et al. Novel mechanism for age-related macular degeneration: an equilibrium shift between the angiogenesis factors VEGF and PEDF. J Cell Physiol 2001;189:323-33.
55. Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A 2009;106:18751-6.
56. Shin Y. J, Nam W.H, Park S.E, Kim J.H, Kim H.K. Aqueous humor concentrations of vascular endothelial growth factor and pigment epithelium-derived factor in high myopic patients. Mol Vis 2012;18:2265-70.
57. Chen W, Song H, Xie S, Han Q, Tang X, Chu Y. Correlation of macular choroidal thickness with concentrations of aqueous vascular endothelial growth factor in high myopia. Curr Eye Res 2015;40:307-13.
58. Yamamoto Y, Miyazaki D, Sasaki S, et al. Associations of inflammatory cytokines with choroidal neovascularization in highly myopic eyes. Retina 2015;35:344-50.
59. Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem 2009;284:6038-42.
60. Bianconi V, Sahebkar A, Atkin SL, Pirro M. The regulation and importance of monocyte chemoattractant protein-1. Curr Opin Hematol 2018;25:44-51.
61. Leveziel N, Yu Y, Reynolds R, et al. Genetic factors for choroidal neovascularization associated with high myopia. Invest Ophthalmol Vis Sci 2012;53:5004-9.
62. Dreismann AK, McClements ME, Barnard AR, et al. Functional expression of complement factor I following AAV-mediated gene delivery in the retina of mice and human cells. Gene Ther 2021;28:265-76.
63. Wang Q, Zhao HS, Li L. Association between complement factor I gene polymorphisms and the risk of age-related macular degeneration: a Meta-analysis of literature. Int J Ophthalmol 2016;9:298-305.
64. Fraczek LA, Martin BK. Transcriptional control of genes for soluble complement cascade regulatory proteins. Mol Immunol 2010;48:9-13.
65. Miyake M, Yamashiro K, Nakanishi H, et al. Evaluation of pigment epithelium-derived factor and complement factor I polymorphisms as a cause of choroidal neovascularization in highly myopic eyes. Invest Ophthalmol Vis Sci 2013;54:4208-12.
66. Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A 1993;90:1526-30.
67. Tombran-tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res 1991;53:411-4.
68. Karakousis P. C, John S.K, Behling K.C, et al. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol Vis 2001;7:154-63.
69. Tombran-tink J, Shivaram S, Chader G, Johnson L, Bok D. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci 1995;15:4992-5003.
70. Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci 2003;4:628-36.
71. Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 1999;285:245-8.
72. Cayouette M, Smith SB, Becerra SP, Gravel C. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol Dis 1999;6:523-32.
73. Wang X, Liu X, Ren Y, et al. PEDF protects human retinal pigment epithelial cells against oxidative stress via upregulation of UCP2 expression. Mol Med Rep 2019;19:59-74.
74. Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem 2006;281:3604-13.
75. Renno R. Z, Youssri A.I, Michaud N, Gragoudas E.S, Miller J.W. Expression of pigment epithelium-derived factor in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2002;43:1574-80.
76. Ho TC, Yang YC, Cheng HC, Wu AC, Chen SL, Tsao YP. Pigment epithelium-derived factor protects retinal pigment epithelium from oxidant-mediated barrier dysfunction. Biochem Biophys Res Commun 2006;342:372-8.
77. Amano S, Yamagishi S, Inagaki Y, et al. Pigment epithelium-derived factor inhibits oxidative stress-induced apoptosis and dysfunction of cultured retinal pericytes. Microvasc Res 2005;69:45-55.
78. Wang Y, Subramanian P, Shen D, Tuo J, Becerra SP, Chan CC. Pigment epithelium-derived factor reduces apoptosis and pro-inflammatory cytokine gene expression in a murine model of focal retinal degeneration. ASN Neuro 2013;5:e00126.
79. Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, García-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res 2019;69:80-115.
80. Ruiz-Medrano J, Flores-Moreno I, Ohno-Matsui K, Cheung CMG, Silva R, Ruiz-Moreno JM. Correlation between atrophy-traction-neovascularization grade for myopic maculopathy and clinical severity. Retina 2021;41:1867-73.
81. Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology 2006;113:2285-91.
82. Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012;119:347-54.
83. Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol 2014;157:451-457.e1.
84. Chia A, Lu QS, Tan D. Five-Year Clinical Trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology 2016;123:391-9.
85. Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci 2012;53:7077-85.
86. Charm J, Cho P. High myopia-partial reduction ortho-k: a 2-year randomized study. Optom Vis Sci 2013;90:530-9.
87. Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol 2009;93:1181-5.
88. Kakita T, Hiraoka T, Oshika T. Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci 2011;52:2170-4.
89. Zhu MJ, Feng HY, He XG, Zou HD, Zhu JF. The control effect of orthokeratology on axial length elongation in Chinese children with myopia. BMC Ophthalmol 2014;14:141.
90. Ng DSC, Fung NSK, Yip FLT, Lai TYY. Ranibizumab for myopic choroidal neovascularization. Expert Opin Biol Ther 2020;20:1385-93.
91. Zhang M, Zhang J, Yan M, et al. KH902 Phase 1 Study Group. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology 2011;118:672-8.
92. Rudge JS, Holash J, Hylton D, et al. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A 2007;104:18363-70.
93. Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE Study Group. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 2014;121:682-92.e2.
94. Chen C, Yan M, Huang Z, Song YP. The evaluation of a two-year outcome of intravitreal conbercept versus ranibizumab for pathological myopic choroidal neovascularization. Curr Eye Res 2020;45:1415-21.
95. Ruiz-Moreno JM, Arias L, Montero JA, Carneiro A, Silva R. Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br J Ophthalmol 2013;97:1447-50.
96. Ng DSC, Ho M, Iu LPL, Lai TYY. Safety review of anti-VEGF therapy in patients with myopic choroidal neovascularization. Expert Opin Drug Saf 2022;21:43-54.
97. Tufail A, Narendran N, Patel PJ, et al. Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology 2013;120:1944-5.e1.
98. Mitchell P, Holz FG, Hykin P, et al. ARIES study investigators. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the aries study: a randomized clinical trial. Retina 2021;41:1911-20.
99. Kim YC, Shin JP, Pak KY, et al. Two-year outcomes of the treat-and-extend regimen using aflibercept for treating diabetic macular oedema. Sci Rep 2020;10:22030.
100. Hamilton RD, Clemens A, Minnella AM, et al. LUMINOUS study group. Real-world effectiveness and safety of ranibizumab for the treatment of myopic choroidal neovascularization: Results from the LUMINOUS study. PLoS One 2020;15:e0227557.
101. Lai TY, Luk FO, Lee GK, Lam DS. Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic choroidal neovascularization. Eye (Lond) 2012;26:1004-11.
102. Cheung CMG, Arnold JJ, Holz FG, et al. Myopic choroidal neovascularization: review, guidance, and consensus statement on management. Ophthalmology 2017;124:1690-711.
103. . Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. Ophthalmology 2001;108:841-52.
104. Virgili G, Menchini F. Laser photocoagulation for choroidal neovascularisation in pathologic myopia. Cochrane Database Syst Rev 2005:CD004765.
105. Iwase T, Ueno Y, Ra E, Ito Y, Terasaki H. Changes in choriocapillaris and retinal morphology after laser photocoagulation by OCT angiography: A case report. Medicine (Baltimore) 2018;97:e13278.
106. Pece A, Brancato R, Avanza P, Camesasca F, Galli L. Laser photocoagulation of choroidal neovascularization in pathologic myopia: long-term results. Int Ophthalmol ;18:339-44.
107. Brancato R, Menchini U, Pece A, Capoferri C, Avanza P, Radrizzani E. Dye laser photocoagulation of macular subretinal neovascularization in pathological myopia. A randomized study of three different wavelengths. Int Ophthalmol 1988;11:235-8.
108. Secrétan M, Kuhn D, Soubrane G, Coscas G. Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser treatment. Eur J Ophthalmol 1997;7:307-316.
109. Brancato R, Pece A, Avanza P, Radrizzani E. Photocoagulation scar expansion after laser therapy for choroidal neovascularization in degenerative myopia. Retina 1990;10:239-43.
110. Parodi M, La Spina C, Berchicci L, Petruzzi G, Bandello F. Photosensitizers and photodynamic therapy: Verteporfin. in: Nguyen Q, Rodrigues E, Farah M, Mieler W, Do D, editors. Retinal Pharmacotherapeutics. S. Karger AG :2015. pp. 330-6.
111. Schlötzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol 2002;240:748-57.
112. . Verteporfin in Photodynamic Therapy (VIP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia. Ophthalmology 2003;110:667-73.
113. Montero JA, Ruiz-Moreno JM. Verteporfin photodynamic therapy in highly myopic subfoveal choroidal neovascularisation. Br J Ophthalmol 2003;87:173-6.
114. Kang HM, Koh HJ. Ocular risk factors for recurrence of myopic choroidal neovascularization: long-term follow-up study. Retina 2013;33:1613-22.
115. Kim YM, Yoon JU, Koh HJ. The analysis of lacquer crack in the assessment of myopic choroidal neovascularization. Eye (Lond) 2011;25:937-46.
116. Zhu Y, Zhang T, Xu G, Peng L. Anti-vascular endothelial growth factor for choroidal neovascularisation in people with pathological myopia. Cochrane Database Syst Rev 2016;12:CD011160.
117. MM Shevelev. Operation against high myopia and scleralectasia with aid of the transplantation of fascia lata on thinned sclera. Russian Oftalmol 1930;11:107-10.
118. WE B. , AA S. Surgical treatment of high myopia; the combined lamellar scleral resection with scleral reinforcement using donor eye. Trans Am Acad Ophthalmol 1958;62:791-802.
120. Nesterov AP, Libenson NB, Svirin AV. Early and late results of fascia lata transplantation in high myopia. Br J Ophthalmol 1976;60:271-2.
121. Chen M, Dai J, Chu R, Qian Y. The efficacy and safety of modified Snyder-Thompson posterior scleral reinforcement in extensive high myopia of Chinese children. Graefes Arch Clin Exp Ophthalmol 2013;251:2633-8.
122. Miao Z, Li L, Meng X, et al. Modified posterior scleral reinforcement as a treatment for high myopia in children and its therapeutic effect. Biomed Res Int 2019;2019:5185780.
123. Dong X, Liu J, Bu J. The efficacy of modified posterior scleral reinforcement with round scleral patches in Chinese children with high myopia. Graefes Arch Clin Exp Ophthalmol 2020;258:1543-7.
124. Xue A, Bao F, Zheng L, Wang Q, Cheng L, Qu J. Posterior scleral reinforcement on progressive high myopic young patients. Optom Vis Sci 2014;91:412-8.
125. Curtin B. J. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc 1977;75:67-86.
126. Curtin BJ, Whitmore WG. Long-term results of scleral reinforcement surgery. Am J Ophthalmol 1987;103:544-8.
127. Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A 2018;115:E7091-100.
128. Liu Y, Wang L, Xu Y, Pang Z, Mu G. The influence of the choroid on the onset and development of myopia: from perspectives of choroidal thickness and blood flow. Acta Ophthalmol 2021;99:730-8.
129. Nickla DL, Totonelly K. Choroidal thickness predicts ocular growth in normal chicks but not in eyes with experimentally altered growth. Clin Exp Optom 2015;98:564-70.
130. Zhou X, Zhang S, Zhang G, et al. Increased choroidal blood perfusion can inhibit form deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci 2020;61:25.
131. Zhang Z, Qi Y, Wei W, et al. Investigation of macular choroidal thickness and blood flow change by optical coherence tomography angiography after posterior scleral reinforcement. Front Med (Lausanne) 2021;8:658259.
132. Frisina R, Gius I, Palmieri M, Finzi A, Tozzi L, Parolini B. Myopic Traction Maculopathy: Diagnostic and management strategies. Clin Ophthalmol 2020;14:3699-708.
133. Ward B, Tarutta EP, Mayer MJ. The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye (Lond) 2009;23:2169-74.
134. Liu B, Ma W, Li Y, et al. Macular buckling using a three-armed silicone capsule for foveoschisis associated with high myopia. Retina 2016;36:1919-26.
135. Alkabes M, Mateo C. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefes Arch Clin Exp Ophthalmol 2018;256:863-77.
136. Parolini B, Frisina R, Pinackatt S, et al. Indications and results of a new l-shaped macular buckle to support a posterior staphyloma in high myopia. Retina 2015;35:2469-82.
137. Liu B, Chen S, Li Y, et al. Comparison of macular buckling and vitrectomy for the treatment of macular schisis and associated macular detachment in high myopia: a randomized clinical trial. Acta Ophthalmol 2020;98:e266-72.
139. Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. J Cataract Refract Surg 2004;30:689-95.
140. Gawargious BA, Le A, Lesgart M, Ugradar S, Demer JL. Differential regional stiffening of sclera by collagen cross-linking. Curr Eye Res 2020;45:718-25.
141. Liu TX, Luo X, Gu YW, Yang B, Wang Z. Correlation of discoloration and biomechanical properties in porcine sclera induced by genipin. Int J Ophthalmol 2014;7:621-5.
142. Lin X, Naidu RK, Dai J, Zhou X, Qu X, Zhou H. Scleral cross-linking using glyceraldehyde for the prevention of axial elongation in the rabbit: blocked axial elongation and altered scleral microstructure. Curr Eye Res 2019;44:162-71.
143. Choi S, Lee SC, Lee HJ, et al. Structural response of human corneal and scleral tissues to collagen cross-linking treatment with riboflavin and ultraviolet A light. Lasers Med Sci 2013;28:1289-96.
144. Krasselt K, Frommelt C, Brunner R, Rauscher FG, Francke M, Körber N. Various cross-linking methods inhibit the collagenase I degradation of rabbit scleral tissue. BMC Ophthalmol 2020;20:488.
145. Wang M, Corpuz CC. Effects of scleral cross-linking using genipin on the process of form-deprivation myopia in the guinea pig: a randomized controlled experimental study. BMC Ophthalmol 2015;15:89.
146. Hannon BG, Luna C, Feola AJ, et al. Assessment of visual and retinal function following in vivo genipin-induced scleral crosslinking. Transl Vis Sci Technol 2020;9:8.
147. Sun M, Zhang F, Li Y, et al. Evaluation of the safety and long-term scleral biomechanical stability of UVA cross-linking on scleral collagen in rhesus monkeys. J Refract Surg 2020;36:696-702.
148. Xue A, Zheng L, Tan G, et al. Genipin-crosslinked donor sclera for posterior scleral contraction/reinforcement to fight progressive myopia. Invest Ophthalmol Vis Sci 2018;59:3564-73.
149. Weiss JN, Levy S. Stem cell ophthalmology treatment study (SCOTS): bone marrow derived stem cells in the treatment of Usher syndrome. Stem Cell Investig 2019;6:31.
150. Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow derived stem cells in the treatment of Dominant Optic Atrophy. Stem Cell Investig 2019;6:41.
151. Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow-derived stem cells in the treatment of Leber's hereditary optic neuropathy. Neural Regen Res 2016;11:1685-94.
152. Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen Res 2015;10:1507-15.
153. Weiss JN, Benes SC, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment. Neural Regen Res 2016;11:1512-6.
154. Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2017;6:493-7.
155. Blasiak J. Senescence in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci 2020;77:789-805.
156. Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet 2016;48:134-43.
157. Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 2016;73:1765-86.
158. Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond) 2016;3:34.
159. Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-16.
160. Ricci F, Bandello F, Navarra P, Staurenghi G, Stumpp M, Zarbin M. Neovascular age-related macular degeneration: therapeutic management and new-upcoming approaches. Int J Mol Sci 2020;21:8242.
161. Tsang SH, Sharma T. Stargardt Disease. In: Tsang SH, Sharma T, editors. Atlas of inherited retinal diseases. Cham: Springer International Publishing; 2018. pp.139-51.
162. Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16-64 years), 1999-2000 with 2009-2010. BMJ Open 2014;4:e004015.
163. Tsybovsky Y, Molday RS, Palczewski K. The ATP-Binding Cassette Transporter ABCA4: Structural and Functional Properties and Role in Retinal Disease. In: Lambris JD, Adamis AP, editors. Inflammation and retinal disease: complement biology and pathology. New York: Springer; 2010. pp. 105-25.
164. Zhang K, Kniazeva M, Han M, et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat Genet 2001;27:89-93.
165. Aveldaño MI. Phospholipid species containing long and very long polyenoic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. Biochemistry 1988;27:1229-39.
166. Suh M, Clandinin MT. 20:5n-3 but not 22:6n-3 is a preferred substrate for synthesis of n-3 very-long- chain fatty acids (C24-C36) in retina. Curr Eye Res 2005;30:959-68.
167. Norris CE, Keener JE, Perera SMDC, et al. Native mass spectrometry reveals the simultaneous binding of lipids and zinc to rhodopsin. Int J Mass Spectrom 2021;460:116477.
168. McMahon A, Jackson SN, Woods AS, Kedzierski W. A Stargardt disease-3 mutation in the mouse Elovl4 gene causes retinal deficiency of C32-C36 acyl phosphatidylcholines. FEBS Lett 2007;581:5459-63.
169. Kniazeva M, Chiang MF, Morgan B, et al. A new locus for autosomal dominant stargardt-like disease maps to chromosome 4. Am J Hum Genet 1999;64:1394-9.
170. Yang Z, Chen Y, Lillo C, et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest 2008;118:2908-16.
171. Stern J, Temple S. Retinal pigment epithelial cell proliferation. Exp Biol Med (Maywood) 2015;240:1079-86.
172. Zhang H, Su B, Jiao L, et al. Transplantation of GMP-grade human iPSC-derived retinal pigment epithelial cells in rodent model: the first pre-clinical study for safety and efficacy in China. Ann Transl Med 2021;9:245.
173. Schwartz SD, Hubschman J, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. The Lancet 2012;379:713-20.
174. Xiang P, Wu KC, Zhu Y, et al. A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials 2014;35:9777-88.
175. Diniz B, Thomas P, Thomas B, et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci 2013;54:5087-96.
176. da Cruz L, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 2018;36:328-37.
177. Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med 2018;10:eaao4097.
178. Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 2017;376:1038-46.
179. Zhang CJ, Ma Y, Jin ZB. The road to restore vision with photoreceptor regeneration. Exp Eye Res 2021;202:108283.
180. Golchin A, Farahany TZ. Biological products: cellular therapy and FDA approved products. Stem Cell Rev Rep 2019;15:166-75.
181. Zhang Z, Wei Y, Jiang X, Zhang S. Pars plana vitrectomy and wide internal limiting membrane peeling with perfluoropropane tamponade for highly myopic foveoschisis-associated macular hole. Retina 2017;37:274-82.
182. Shao Q, Xia H, Heussen FM, Ouyang Y, Sun X, Fan Y. Postoperative anatomical and functional outcomes of different stages of high myopia macular hole. BMC Ophthalmol 2015;15:93.
183. Qu J, Zhao M, Jiang Y, Li X. Vitrectomy outcomes in eyes with high myopic macular hole without retinal detachment. Retina 2012;32:275-80.
184. Fang Y, Yokoi T, Shimada N, et al. Development of macular atrophy after pars plana vitrectomy for myopic traction maculopathy and macular hole retinal detachment in pathologic myopia. Retina 2020;40:1881-93.
185. Alkabes M, Pichi F, Nucci P, et al. Anatomical and visual outcomes in high myopic macular hole (HM-MH) without retinal detachment: a review. Graefes Arch Clin Exp Ophthalmol 2014;252:191-9.
186. Pang CE, Sarraf D, Freund KB. Extreme choroidal thinning in high myopia. Retina 2015;35:407-15.
187. Keeley F, Morin J, Vesely S. Characterization of collagen from normal human sclera. Exp Eye Res 1984;39:533-42.
188. Boote C, Sigal IA, Grytz R, Hua Y, Nguyen TD, Girard MJA. Scleral structure and biomechanics. Prog Retin Eye Res 2020;74:100773.
190. Shen L, You QS, Xu X, et al. Scleral and choroidal volume in relation to axial length in infants with retinoblastoma versus adults with malignant melanomas or end-stage glaucoma. Graefes Arch Clin Exp Ophthalmol 2016;254:1779-86.
191. Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera of high myopia. An electron microscopic study. Arch Ophthalmol 1979;97:912-5.
192. Guggenheim J. A, McBrien N.A. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci 1996;37:1380-95.
193. Rada J. A, Nickla D.L, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci ;41:2050-8.
194. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 2003;200:500-3.
195. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001;12:2730-41.
196. Jobling AI, Gentle A, Metlapally R, McGowan BJ, McBrien NA. Regulation of scleral cell contraction by transforming growth factor-beta and stress: competing roles in myopic eye growth. J Biol Chem 2009;284:2072-9.
197. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349-63.
198. Shinohara K, Yoshida T, Liu H, et al. Establishment of novel therapy to reduce progression of myopia in rats with experimental myopia by fibroblast transplantation on sclera. J Tissue Eng Regen Med 2018;12:e451-61.
199. Tsai CL, Wu PC, Fini ME, Shi S. Identification of multipotent stem/progenitor cells in murine sclera. Invest Ophthalmol Vis Sci 2011;52:5481-7.
200. Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res 2000;2:477-88.
201. Nakahara H. In vitro differentiation of bone and hypertrophic cartilage from periosteal-derived cells*1. Exp Cell Res 1991;195:492-503.
202. Čamernik K, Marc J, Zupan J. Human skeletal muscle-derived mesenchymal stem/stromal cell isolation and growth kinetics analysis. In: Turksen K, editor. Stem Cells and Aging. New York: Springer; 2019. pp. 119-29.
203. Friedenstein A. J., Gorskaja J.F., Kulagina N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 1976;4:267-74. [PMID:976387.
204. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000;97:13625-30.
205. Wang L, Huang S, Li S, et al. Efficacy and safety of umbilical cord mesenchymal stem cell therapy for rheumatoid arthritis patients: a prospective phase I/II study. Drug Des Devel Ther 2019;13:4331-40.
206. In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003;102:1548-9.
207. In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004;22:1338-45.
208. Motaln H, Schichor C, Lah TT. Human mesenchymal stem cells and their use in cell-based therapies. Cancer 2010;116:2519-30.
209. Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005;105:2821-7.
210. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22.
211. Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006;107:367-72.
212. Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005;105:2214-9.
213. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076-84.
214. Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009;20:419-27.
215. Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004;109:1543-9.
216. Seah I, Liu Z, Soo Lin Wong D, et al. Retinal pigment epithelium transplantation in a non-human primate model for degenerative retinal diseases. J Vis Exp 2021; doi: 10.3791/62638.
217. Li XJ, Yang XP, Li QM, et al. Posterior scleral reinforcement for the treatment of pathological myopia. Int J Ophthalmol 2016;9:580-4.
218. Peng C, Xu J, Ding X, et al. Effects of posterior scleral reinforcement in pathological myopia: a 3-year follow-up study. Graefes Arch Clin Exp Ophthalmol 2019;257:607-17.
219. Shen ZM, Zhang ZY, Zhang LY, Li ZG, Chu RY. Posterior scleral reinforcement combined with patching therapy for pre-school children with unilateral high myopia. Graefes Arch Clin Exp Ophthalmol 2015;253:1391-5.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ma Y, Li YP, Jin ZB. Stem cell-based therapy for myopic maculopathy: a new concept. J Transl Genet Genom 2022;6:179-203. http://dx.doi.org/10.20517/jtgg.2021.48
AMA Style
Ma Y, Li YP, Jin ZB. Stem cell-based therapy for myopic maculopathy: a new concept. Journal of Translational Genetics and Genomics. 2022; 6(2): 179-203. http://dx.doi.org/10.20517/jtgg.2021.48
Chicago/Turabian Style
Ma, Ya, Yan-Ping Li, Zi-Bing Jin. 2022. "Stem cell-based therapy for myopic maculopathy: a new concept" Journal of Translational Genetics and Genomics. 6, no.2: 179-203. http://dx.doi.org/10.20517/jtgg.2021.48
ACS Style
Ma, Y.; Li Y.P.; Jin Z.B. Stem cell-based therapy for myopic maculopathy: a new concept. J. Transl. Genet. Genom. 2022, 6, 179-203. http://dx.doi.org/10.20517/jtgg.2021.48
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 14 clicks
Cite This Article 14 clicks



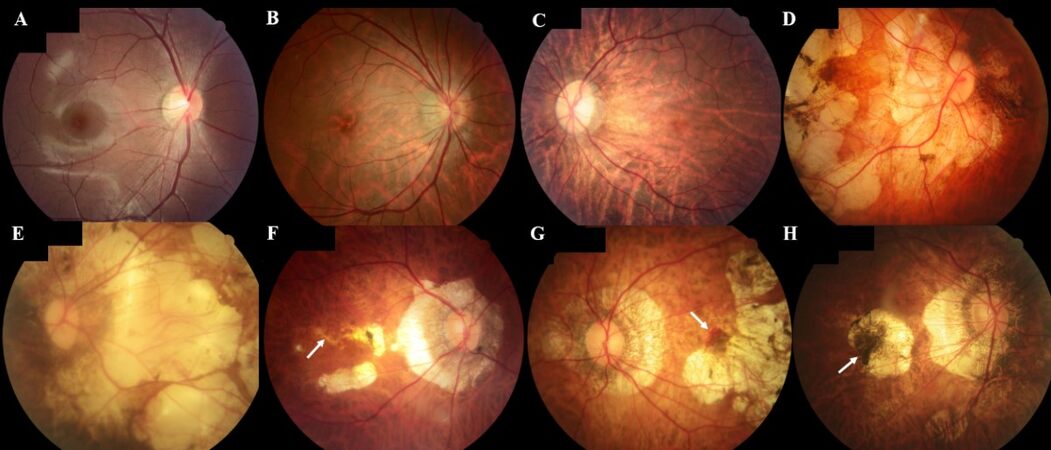
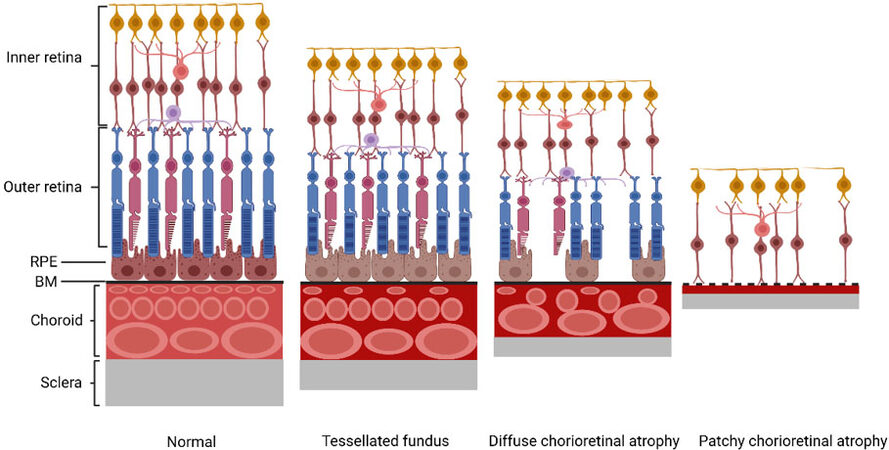
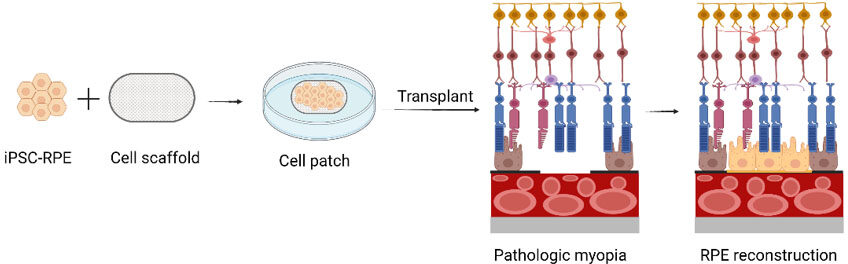
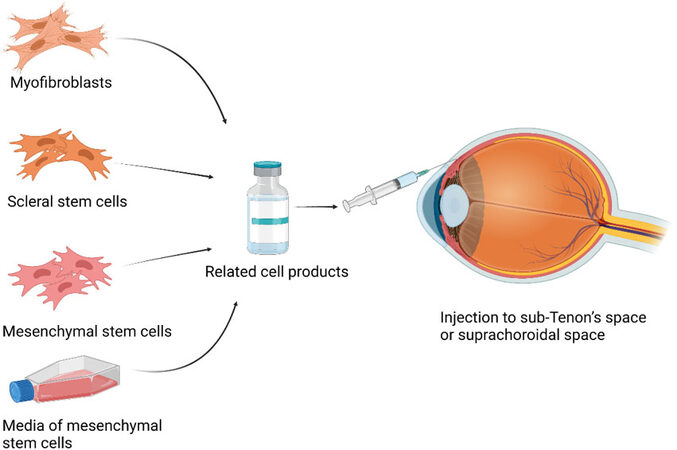









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.