What to do with patients with active infective endocarditis complicated by intracranial bleeding
Abstract
Cerebral complications, especially intracranial hemorrhage (ICH), are critical determinants of the early outcomes of cardiac surgery for active infective endocarditis (AIE). Relevant society guidelines still recommend delaying cardiac surgery for AIE complicated by ICH for 4 weeks. Some early studies indicated that the mortality decreases when cardiac surgery for ICH is delayed. In contrast, some reported that surgical intervention should not be delayed if an early operation is indicated, even in patients with ICH. The current literature on early versus late surgery for AIE with ICH is conflicting. ICH is classified by its mechanism which includes primary intraparenchymal hemorrhage, hemorrhagic transformation of ischemic infarcts, and rupture of intracranial infectious aneurysms. Some reported that for AIE with a mycotic cerebral aneurysm, early cardiac surgery should be done after repair of the aneurysm, either surgically or endovascularly. Except for the rupture of mycotic aneurysm, primary intraparenchymal hemorrhage and hemorrhagic transformation of ischemic infarcts remain a critical and challenging dilemma. Modifying the cardiopulmonary bypass (CPB) strategy might be necessary to improve the surgical outcomes of AIE with ICH. Some studies reported that cardiac surgery using nafamostat mesylate as an alternative anticoagulant during CPB (NM-CPB) was performed successfully. The NM-CPB can be a useful option as an anticoagulant in critical situations of cardiac surgery with ICH. The timing of surgery should be decided on a case-by-case basis with multidisciplinary specialties including cardiac and neurological teams.
Keywords
INTRODUCTION
Cerebral complications are critical determinants of the in-hospital outcomes of cardiac surgery for active infective endocarditis (AIE). Intracranial hemorrhage (ICH) is a major potential risk factor for worse outcomes in patients who are undergoing open cardiac surgery for AIE. Delaying cardiac surgery for patients with ICH may exacerbate local or systemic infections. On the other hand, early cardiac surgery using normal heparin doses of systemic anticoagulation carries the risk of further bleeding and neurologic deterioration. Therefore, AIE with ICH has a critical and challenging dilemma. In this report, the current literature on cardiac surgery for patients with AIE and ICH was reviewed and discussed.
METHODS
We systematically searched MEDLINE without language restrictions for relevant studies using the following Medical Subject Headings (MeSH) terms present in either the title or abstract: “endocarditis”, “cardiac surgery”, “stroke”, “intracranial complications”, and “intracranial hemorrhages”. Our search strategy was that eligible studies had to fulfill the following criteria for inclusion: (1) cohort study, either prospective or retrospective; (2) patients receive a definite diagnosis of AIE according to the modified Duke criteria[1]; (3) the study treated early surgery for AIE patients with intracranial complications, including late surgery (we defined early surgery as surgery performed within 2 weeks from the onset of the neurological event[2]); and (4) the study investigated short-term and long-term efficacy and neurological exacerbation as outcomes. After screening titles and abstracts, 183 clinical studies were found and analyzed.
Current guidelines
Current guidelines for AIE stated that cardiac surgery should be considered without any delay in patients with heart failure, uncontrolled infection, annular abscess, or persistent high-risk of systemic embolism, except for patients with coma, brain hernia, or large major central lesions[2-5][Table 1]. However, postponing the cardiac surgery for AIE with ICH for four weeks is still recommended. As for a relationship of neurological outcomes and the size of the brain lesions of stroke or ICH secondary to AIE, no study has ever been published.
Guidelines and indication for cardiac surgery for the patients with intracranial hemorrhage
| COR | LOE | ||
| ESC 2015[3] | Following intracranial hemorrhages, surgery should be generally postponed for > 1 month | IIb | B |
| AATS 2017[4] | In patients with a recent intracranial hemorrhage, a delay in operation for three or more weeks is reasonable | IIb | B |
| ACC/AHA 2017[2] | Delaying the valve surgery for at least four weeks may be considered for patients with IE and major ischemic stroke or intracranial hemorrhage if hemodynamically stable | IIb | B |
| JCS 2019[5] | Surgery should be postponed for at least four weeks when a new intracranial hemorrhage is detected Note: Except for cases involving cerebral microbleeds | IIb | B |
Early studies
There have been many earlier studies regarding treating AIE patients with cerebral complications. In 1995, Eishi et al. analyzed 34 patients with ICH and reported that the risk of neurological deterioration is still significantly high 15 days and even four weeks after cerebral hemorrhage[6]. In 1996, Gillinov et al. reported good outcomes in 7 patients with AIE and ICH, in whom the surgery was postponed for 2 to 3 weeks[7]. In 2014, Wilbring et al. reported that a neurological deterioration was found in 33% of 6 patients with AIE and ICH who underwent surgery after 17 ± 24 days of diagnosis[8]. In 2006, Ruttmann et al. reported a mortality of 66% after an early surgery of patients with ICH[9]. In 2013, Garcia-Cabrera et al. reported that the mortality of patients with AIE and ICH who had surgery within 14 days was 75%, and that neurological exacerbation occurred in 50%[10]. In 2016, A multi-center registry data analyzing the AIE patients complicated with stroke or ICH by Okita et al. demonstrated that patients with AIE and ICH who underwent open cardiac surgery had higher mortality when the surgery was performed within seven days after AIE and ICH onset than those who underwent surgery 8-21 days after the onset[11]. These studies indicate that the mortality rate decreases when cardiac surgery for patients with ICH is delayed.
Recent studies
Conversely, recent studies by Shang et al. reported that cardiac surgery on 16 patients with AIE and ICH within four days after admission whose ICH size was smaller than 1-2 cm was safely performed[12].
Summary of the studies of cardiac surgery for infective endocarditis with preoperative ICH
| Authors | Study design | No. PTs with ICH | Onset to surgery, median | Neurological exacerbtion | Mortality | Authors’ comments |
| Eishi et al.[6] | Retro | ICH 34 | < 24 h (n = 1) 5 days ( n = 1) 15-21 days (n = 5) 22-28 days (n = 6) > 4 weeks (n = 210) | < 24 h:100% > 4 weeks:19% 2-28 days: 0% | < 24 h: 100% 5 days: 0% 15-21 days: 20% 22-28 days: 0% > 4 weeks: 19% | There is some risk of progression of cerebral damage 15 days and even 4 weeks after the hemorrhage, regardless of the timing of the operation. Reduced heparization in conjunction with a heparin-coated pump system would useful during cardiac operations |
| Ruttmann et al.[9] | Retro | ICH 6 | 4.0 days | 4 Pts | 66.5% (4/6) | |
| Garcia-Cabrer et al.[10] | Retro | ICH 12 | < 14 days (n = 4) 14-21 days (n = 3) > 21 days (n = 5) | < 14 days 75% 14-21 days 66%, > 21 days 40% | 58.3% (7/12) | In patients with cerebral hemorrhage, it may be advisable to postpone cardiac surgery for > 4 weeks |
| Okita et al.[11] | Retro | ICH 54 | < 7 days (n = 13) 8-21 days (n = 17) > 21 days (n = 24) | < 7 days: 15.4% 8-21 days: 5.9% > 21 days: 0% | Very early surgery (within 7 days) should be avoided in patients with ICH | |
| Shang et al.[12] | Retro | ICH16 | 4.0 days (admission to surgery) | 0 | 0 | We do not delay cardiac surgery because of ICH that does not exceed 1 or 2 cm in size |
| Yoshioka et al.[13] | Retro | ICH 30 | < 7 days (n = 5) 8-14 days (n = 6) 15-28 days (n = 9) > 29 days (n = 10) | 0 | 0 | No neurologic deterioration, regardless of surgical timing |
| Kume et al.[14] | Retro | ICH 25 | < 14 days (n = 17) > 14 days (n = 8) | 4.0% (n = 1) | 4.0% (n = 1) | No significant difference in post-operative hemorrhage between pts within or after 14 days |
| Tam et al.[15] | Retro. meta-analysis | ICH 38 | < 4 weeks (n = 17) Median 34 days | 18.8% | 15.8% | The Pts with ICH might benefit from delayed surgery beyond 21 days |
Insights from neurology
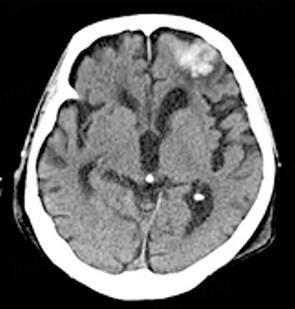
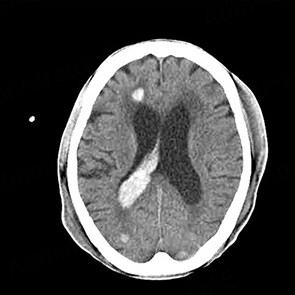
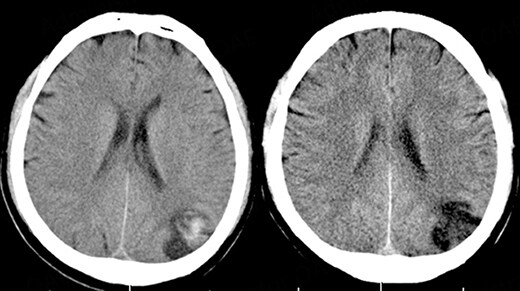
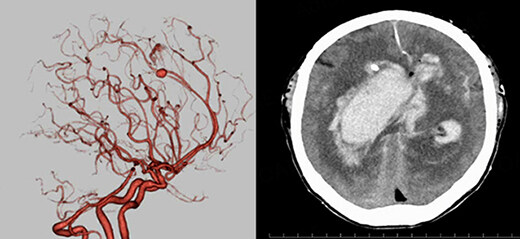
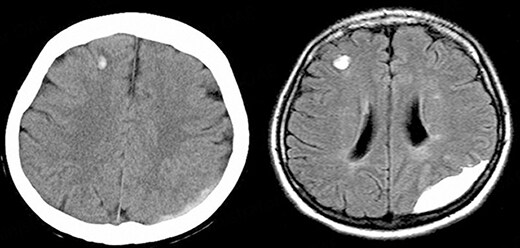
ICH is classified into primary intraparenchymal hemorrhage [Figures 1 and 2], hemorrhagic transformation of ischemic infarcts [Figure 3], and rupture of intracranial infectious aneurysms [Figure 4][16]. Primary intraparenchymal hemorrhage is due to the septic erosion of the arterial wall with rupture. For non-IE primary intraparenchymal hemorrhage, several scoring systems were developed for predicting long-term outcomes. Many factors, such as lesion size, lesion location, neurologic function at presentation, age, and pre-morbid functional status may contribute to patients’ outcomes. An ICH with a volume of 30 mL, measured by computerized tomography (CT) scan, and intraventricular/infratentorial ICH tend to be associated with a worse prognosis[17,18]. The hemorrhagic transformation of ischemic infarcts can be attributed to dysfunctions in the blood-brain barrier and the degradation of the extracellular matrix of the brain[19]. While mycotic aneurysm is not included in the category of ICH, these lesions are common in AIE patients. They are considered to be a potential cause of subarachnoid hemorrhage [Figure 5]. In patients with cerebral aneurysms, neuro-surgical or endovascular intervention prior to cardiac surgery should be strongly considered[20,21]. A recent study by Salaun et al. showed that patients with an undetermined etiology of ICH had significantly higher mortality compared to patients with ICH secondary to ruptured mycotic aneurysm or ischemic stroke[22].
Figure 3. 66-year-old male with aortic valve infective endocarditis. Hemorrhagic transformation of stroke after surgery.
Figure 4. 74-year-old male with aortic valve infective endocarditis. Cerebral mycotic aneurysm ruptured.
Innovations in cardiopulmonary bypass (CPB) strategy
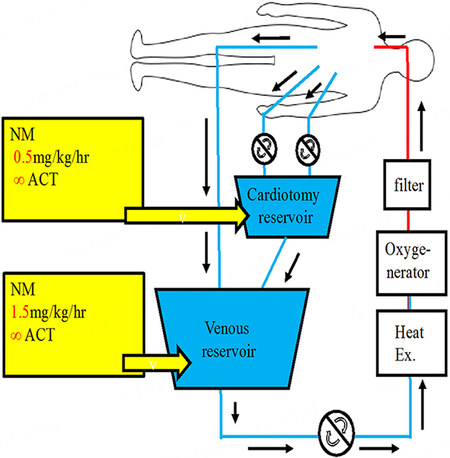
During a conventional CPB using a normal conventional dose of heparin, the coagulo-fibrinolytic system is greatly activated[23]. Modifying the CPB strategy might be necessary to improve the surgical outcomes for AIE with ICH. Some authors have reported that using low-dose heparin (100 IU/kg) during cardiac surgery resulted in reduced blood loss and transfusion requirements[24,25]. We reported the advantages of open cardiac surgery using nafamostat mesylate (NM; 6-amino-2 naphthalene-p-guanidinobenzoate dimethanesulfonate) as an alternative anticoagulant during CPB (NM-CPB) to reduce ICH exacerbation in AIE patients with stroke or ICH[26,27]. NM has a strong inhibitory activity on various proteases in the coagulo-fibrinolytic system. Although its exact mechanism has not been elucidated, NM has a very short half-time and has potential inhibitory effects on the coagulation, fibrinolysis, and platelet aggregation system. Our anticoagulation protocol during NM-CPB has been that NM (1.0 mg/kg) was administered intravenously to assess for any allergic reaction after induction of anesthesia. An initial dose of heparin
Figure 6. Methods of cardiopulmonary bypass using nafamostat mesylate (NM-CPB). Shown in the image is the pump circuit of NM-CPB. Nafamostat mesylate was infused continuously to the cardiotomy reservoir and venous reservoir at 0.8 mg/kg/h and 1.0 mg/kg/h, respectively. The rate of injection was changed according to the activated clotting time (ACT). Heparin: 50 IU/kg (25% of normal), ACT: 350-450 q 15 min, Mild hypothermia: (33 °C), Pump flow: 2.4-2.6 L/m2/min, Systemic pressure: 50 mmHg.
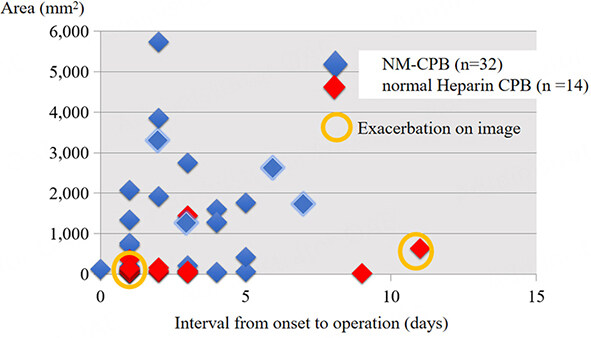
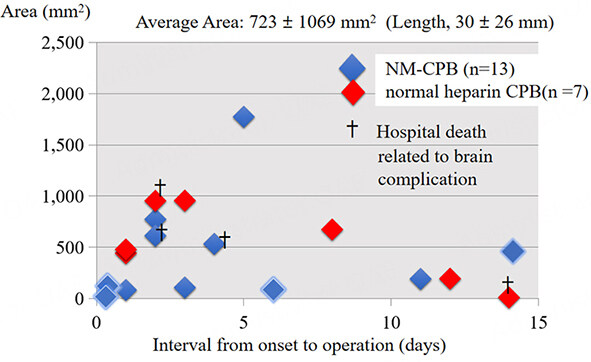
We showed the relationships between the size of infarction or hemorrhage and the timing of cardiac surgery for patients who underwent NM-CPB. Regarding acute stroke [Figure 7], in two patients who had normal CPB using the conventional dose of heparin, a hemorrhagic transformation of the brain lesions occurred postoperatively. However, another patient who had a stroke over 3000 mm2 in size, had a successful early cardiac surgery without any exacerbation of stroke. As for ICH [Figure 8], there were four hospital death related to brain complications in two with NM-CPB and two with normal heparin CPB. The hospital mortality in patients with NM-CPB, and patients with normal heparin CPB were 15.3% and 28.5%, respectively. Among patients who underwent surgeries within seven days from the onset of the AIE, mortality in patients with NM-CPB and with normal heparin CPB were 16.6% (2/12), and 33.3% (1/3), respectively. This might indicate that an early surgery for ICH could be performed more safely by NM-CPB.
Figure 7. Relationships between the size of acute cerebral infarction and the interval from onset to operation (total
Figure 8. Relationships between the size of intracranial hemorrhage and the interval from onset to operation (total n = 20, NM-CPB
CONCLUSIONS
The current guidelines still recommend delaying cardiac surgery for patients with AIE with ICH for four weeks. Understanding the spectrum of ICH with AIE based on the mechanism of hemorrhage, early cardiac surgery for the AIE with mycotic aneurysm may be safely done by preceding surgical or endovascular repair for cerebral aneurysm. NM is a useful option as an anticoagulant during the open cardiac surgery with ICH. The timing of surgery should be decided on a case-by-case basis with multidisciplinary specialties, consisting of cardiac and neurologic teams.
DECLARATIONS
Authors’ contributionsWrote the manuscript: Yamazato T
Carried out the trial and analyzed data: Okada K, Munakata H
Supervised the project: Okita Y
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8.
2. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185.
3. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J 2015;36:3075-128.
4. Pettersson GB, Coselli JS, Pettersson GB, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines. J Thorac Cardiovasc Surg 2017;153:1241-1258.e29.
5. Nakatani S, Ohara T, Ashihara K, et al. JCS 2017 guideline on prevention and treatment of infective endocarditis. Circ J 2019;83:1767-809.
6. Eishi K, Kawazoe K, Kuriyama Y, Kitoh Y, Kawashima Y, Omae T. Surgical management of infective endocarditis associated with cerebral complications. J Thorac Cardiovasc Surg 1995;110:1745-55.
7. Gillinov AM, Shah RV, Curtis WE, et al. Valve replacement in patients with endocarditis and acute neurologic deficit. Ann Thorac Surg 1996;61:1125-30.
8. Wilbring M, Irmscher L, Alexiou K, Matschke K, Tugtekin SM. The impact of preoperative neurological events in patients suffering from native infective valve endocarditis. Interact Cardiovasc Thorac Surg 2014;18:740-7.
9. Ruttmann E, Willeit J, Ulmer H, et al. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke 2006;37:2094-9.
10. García-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis. Circulation 2013;127:2272-84.
11. Okita Y, Minakata K, Yasuno S, et al. Optimal timing of surgery for active infective endocarditis with cerebral complications: a Japanese multicentre study. Eur J Cardiothorac Surg 2016;50:374-82.
12. Shang E, Forrest GN, Chizmar T, et al. Mitral valve infective endocarditis: benefit of early operation and aggressive use of repair. Ann Thorac Surg 2009;87:1728-33.
13. Yoshioka D, Toda K, Sakaguchi T, et al. Valve surgery in active endocarditis patients complicated by intracranial haemorrhage. Eur J Cardiothorac Surg 2014;45:1082-8.
14. Kume Y, Fujita T, Fukushima S, et al. Intracranial mycotic aneurysm is associated with cerebral bleeding post-valve surgery for infective endocarditis. Interact Cardiovasc Thorac Surg 2018;27:635-41.
15. Tam DY, Yanagawa B, Verma S, et al. Early vs late surgery for patients with endocarditis and neurological injury. Can J Cardiol 2018;34:1185-99.
16. Hart RG, Kagan-hallet K, Joerns SE. Mechanisms of intracranial hemorrhage in infective endocarditis. Stroke 1987;18:1048-56.
17. Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage. Stroke 2008;39:2304-9.
18. Parry-Jones AR, Abid KA, Di Napoli M, et al. Accuracy and clinical usefulness of intracerebral hemorrhage grading scores: a direct comparison in a UK population. Stroke 2013;44:1840-5.
19. Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol 2013;4:32.
20. Piper C, Wiemer M, Schulte HD, Horstkotte D. Stroke is not a contraindication for urgent valve replacement in acute infective endocarditis. J Heart Valve Dis 2001;10:703-11.
21. Maruyama R, Yamada A, Sugiyama T, et al. Mitral valve repair for endocarditis can be performed 3 days after repair of a bleeding mycotic brain aneurysm. J Thorac Cardiovasc Surg 2016;151:e59-61.
22. Salaun E, Touil A, Hubert S, et al. Intracranial haemorrhage in infective endocarditis. Arch Cardiovasc Dis 2018;111:712-21.
23. Tanaka K, Morimoto T, Yada I, Kusagawa M, Deguchi K. Physiologic role of enhanced fibrinolytic activity during cardiopulmonary bypass in open heart surgery. ASAIO Trans 1987;33:505-9.
24. Segesser LK, Weiss BM, Pasic M, Garcia E, Turina MI. Risk and benefit of low systemic heparinization during open heart operations. Ann Thorac Surg 1994;58:391-8.
25. Øvrum E, Brosstad F, Åm Holen E, Tangen G, Abdelnoor M. Effects on coagulation and fibrinolysis with reduced versus full systemic heparinization and heparin-coated cardiopulmonary bypass. Circulation 1995;92:2579-84.
26. Sakamoto T, Kano H, Miyahara S, et al. Efficacy of nafamostat mesilate as anticoagulation during cardiopulmonary bypass for early surgery in patients with active infective endocarditis complicated by stroke. J Heart Valve Dis 2014;23:744-51.
27. Ota T, Okada K, Kano H, Okita Y. Cardiopulmonary bypass using nafamostat mesilate for patients with infective endocarditis and recent intracranial hemorrhage. Interact Cardiovasc Thorac Surg 2007;6:270-3.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yamazato T, Munakata H, Okada K, Okita Y. What to do with patients with active infective endocarditis complicated by intracranial bleeding. Vessel Plus 2023;7:2. http://dx.doi.org/10.20517/2574-1209.2022.01
AMA Style
Yamazato T, Munakata H, Okada K, Okita Y. What to do with patients with active infective endocarditis complicated by intracranial bleeding. Vessel Plus. 2023; 7: 2. http://dx.doi.org/10.20517/2574-1209.2022.01
Chicago/Turabian Style
Yamazato, Takahiro, Hiroshi Munakata, Kenji Okada, Yutaka Okita. 2023. "What to do with patients with active infective endocarditis complicated by intracranial bleeding" Vessel Plus. 7: 2. http://dx.doi.org/10.20517/2574-1209.2022.01
ACS Style
Yamazato, T.; Munakata H.; Okada K.; Okita Y. What to do with patients with active infective endocarditis complicated by intracranial bleeding. Vessel Plus. 2023, 7, 2. http://dx.doi.org/10.20517/2574-1209.2022.01
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 13 clicks
Cite This Article 13 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.