Postoperative atrial fibrillation is associated with increased resource utilization after cardiac surgery: a regional analysis of the Southeastern United States
Abstract
Aim: Postoperative atrial fibrillation (POAF) is a known risk factor for morbidity and mortality following cardiac surgery though contemporary resource utilization data is limited. We hypothesize that POAF increases the length of stay, hospital cost, and discharges to facilities, though this trend may be tempering over time.
Methods: Records were extracted for all patients in a regional database who underwent coronary artery bypass grafting, aortic valve replacement, or both (2012-2020). Patients without a history of atrial fibrillation were stratified by POAF for univariate analysis. Patients were propensity-score matched to account for baseline, operative, and postoperative differences.
Results: Of the 27,307 cardiac surgery patients, 23% developed POAF. Matching resulted in 5926 well-balanced pairs of patients with and without POAF. Every metric of resource utilization was higher for patients with POAF, including ICU length of stay (58 h vs. 49 h, P < 0.0001), postoperative length of stay (7 days vs. 5 days, P < 0.0001), discharge to a facility (27% vs. 23%, P < 0.0001), and readmission (11% vs. 8%). The mean additional total hospital cost attributable to POAF was $6705 by paired analysis. A sensitivity analysis of only patients without major complications demonstrated similarly increased resource utilization for patients with POAF.
Conclusions: POAF was associated with an increased 9 additional ICU hours, 2 postoperative days, 18% more discharges to a facility, and 33% greater readmissions. An additional $6705 is associated with POAF. These conservative estimates demonstrate the broad impact of POAF on in and out of hospital resource utilization that warrants future efforts at containment and quality improvement.
Keywords
INTRODUCTION
Postoperative atrial fibrillation (POAF) is one of the most common complications after cardiac surgery. For coronary artery bypass graft (CABG) patients the incidence is approximately 25%-30%, while after aortic valve replacement (AVR), the incidence is 30%-40%, and combined CABG/AVR has the highest incidence at over 35%[1]. Onset of POAF is a time-related hazard with peak incidence on postoperative day two that declines over the following week[2]. The causes of POAF are multifactorial and include preoperative structural changes and perioperative proarrhythmic adrenergic activation, inflammation, oxidative stress, and electrolyte derangement[1,3,4].
There is clear evidence that POAF increases morbidity and resource utilization. However, there are considerable limitations to the estimations. The studies are retrospective, observational studies with all the limitations that apply. Assessments of resource utilization largely utilize preoperative risk factors and risk scores for adjustment. Finally, most do not account for other complications known to also increase resource utilization[5]. There is significant co-linearity of POAF and other complications that obscure a clear delineation of the true impact of POAF. However, since prior estimates of total healthcare costs of POAF were $1 billion back in 2008, continued efforts to update and improve resource utilization estimates are warranted[6].
The Virginia Cardiac Services Quality Initiative (VCSQI) is a regional consortium of hospitals that uniquely includes both clinical and cost data. This large cohort of patients represents an ideal opportunity to further clarify potential associations of POAF with complications and resource utilization. We hypothesized that POAF is associated with increased morbidity and resource utilization. Furthermore, we believe that efforts to decrease POAF may have limited the increase in resource utilization over time as compared with overall cost trends in cardiac surgery.
METHODS
Patient data
The VCSQI is a regional collaborative of 18 hospitals in Virginia. Hospitals submit clinical data using the Society of Thoracic Surgeons (STS) data entry forms. Cost data is submitted using Uniform Billing-04 forms which include final hospital charges. Clinical and cost data are merged at the patient level. Charges are classified by the International Classification of Diseases revenue codes into cost buckets. The charges are then multiplied by corresponding cost-to-charge ratios. Cost data were then adjusted for medical inflation using the market basket for the Centers for Medicare and Medicaid Services Inpatient Prospective Payment System, and presented in 2020 dollars[7].
Data use and business associate agreements are in place with all members, VCSQI and the database vendor (ARMUS Corporation, Foster City, CA). The primary objective of the VCSQI is quality improvement, including prior work on the prevention of POAF. As this analysis represents a secondary analysis of the registry without Health Insurance Portability and Accountability Act identifiers, it is exempt from Institutional Review Board review per University of Virginia IRB policy.
De-identified records for all isolated CABG, AVR, and CABG/AVR patients from January 2012 through December 2020 were extracted from the VCSQI data registry. Patients were excluded for missing atrial fibrillation status, preoperative risk scores, and missing or zero charge data. Patients with arrhythmias at baseline were excluded. A subgroup analysis was performed, excluding patients with major complications after surgery (STS major morbidity). All clinical variables utilize standard STS definitions, including operative mortality (30-day or in-hospital mortality) and major morbidity (permanent stroke, prolonged ventilation, reoperation for any reason, renal failure, and deep sternal wound infection)[8].
Statistical analysis
Categorical variables are presented as counts (%) and continuous variables as median [25th, 75th percentile]. Cost data is presented as both median [25th, 75th percentile] and mean ± standard deviation (SD) to best clarify total cost implications. Patients were stratified by POAF for univariate analysis using the Chi-square test for categorical variables and Mann-Whitney U-test for continuous variables. Data missingness was low, no imputation was used for this first set of analyses, and missing data points were excluded from the corresponding analysis. Henceforth this group will be called the pre-match cohort.
To account for baseline and postoperative differences, patients were propensity-score matched by POAF status. Data missingness was accounted for with simple imputations where variables with < 5% missing data were imputed using the methodology described in the creation of the STS risk models[9]. This includes the lower risk category for categorical variables and the median for continuous variables, with gender-specific medians for body surface area. Next, propensity scores were created using logistic regression and 35 variables, including demographics, preoperative risk factors, and postoperative complications
RESULTS
Patient and operative characteristics
A total of 37,676 patients underwent CABG and/or AVR, of whom 1344 (3.63%) had a history of atrial fibrillation and were excluded. After additional exclusion of patients with other documented preoperative arrhythmia, as well as those lacking data for STS predicted risk of mortality, cost data, year of surgery, or POAF, 27,307 patients were identified for analysis. Of these 27,307 patients, 6315 (23.1%) developed POAF
Patients who developed POAF were older (70 median years of age vs. 64 median years of age, P < 0.001), less likely to be women (25.0% vs. 27.7%, P < 0.001), and, in general, had a higher burden of comorbid disease including hypertension (89.1% vs. 86.4%, P < 0.001), diabetes (44.3% vs. 47.1%, P < 0.001), moderate to severe chronic lung disease (12.2 % vs. 9.92%, P < 0.001), and heart failure (31.7% vs. 28.2%, P < 0.001). Patients who developed POAF were less likely to receive a preoperative beta-blocker, relative to those who did not develop POAF (86.7% vs. 88.2%, P = 0.002). STS predicted risk of mortality was significantly greater for patients who developed POAF (1.46% vs. 1.02%, P < 0.001).
Patients developing POAF were less likely to receive surgery on an emergent or urgent basis, relative to those not developing POAF (52.9% vs. 57.8%, P < 0.001). A significantly greater proportion of POAF patients had an IABP placed during their hospitalization, relative to those without POAF (7.68% vs. 6.44%, P < 0.001). Rates of POAF did vary significantly by procedure, and the number of disease coronary arteries present (P < 0.001 for both). Patients with POAF underwent longer cross-clamp (75.0 min vs. 70.0 min, P < 0.001) and cardiopulmonary bypass times (101 min vs. 94.0 min, P < 0.001), relative to those without POAF.
Postoperative outcomes and resource utilization
By univariate analysis POAF was associated with significantly increased rates of STS operative mortality (3.04% vs. 1.43%, P < 0.001), and major morbidity (13.7% vs. 6.07%, P < 0.001). Incidence of cardiac arrest (2.95% vs. 1.02%, P < 0.001), pneumonia (3.71 vs. 1.15, P < 0.001), and transfusion requirement (34.4% vs. 21.5%, P < 0.001) was elevated among patients who developed POAF relative to those who did not
POAF was also associated with significantly increased resource utilization. On univariate analysis postoperative length of stay (7.0 days vs. 5.0 days, P < 0.001), intensive care unit (ICU) length of stay (67.0 h vs. 46.0 h, P < 0.001), discharge to facility (29.2% vs. 16.6%, P < 0.001), and readmission (11.7% vs. 8.15%, P < 0.001) were all significantly increased among patients with POAF vs. those without POAF. Total hospital costs were significantly increased for patients who developed POAF, relative to those who did not ($42,324 vs. $36,682, P < 0.001).
Propensity matched baseline and operative characteristics
A total of 5926 pairs of patients were matched between POAF and no POAF patients. These were well matched with all baseline covariates having a standardized mean difference of < 10% [Table 1, Supplementary Figures 1 and 2]. Median predicted risk of mortality was similar between both groups of matched patients (1.32% vs. 1.29%, P = 0.505).
Baseline and operative characteristics by POAF status of the matched cohort
| POAF (n = 5926) | No POAF (n = 5926) | SMD | P-value | |
| Age | 69.0 (63.0-75.0) | 69.0 (63.0-75.0) | 0.01 | 0.716 |
| BSA | 2.10 (1.92-2.27) | 2.09 (1.92-2.26) | 0.00 | 0.449 |
| Female | 25.0 (1483) | 25.6 (1514) | 0.01 | 0.534 |
| Hypertension | 88.9 (5267) | 88.6 (5251) | 0.01 | 0.853 |
| Diabetes | 44.6 (2642) | 44.2 (2617) | 0.01 | 0.781 |
| Dialysis dependent renal failure | 2.73 (162) | 2.82 (167) | 0.01 | 0.337 |
| Prior stroke | 8.49 (503) | 7.75 (459) | 0.02 | 0.235 |
| Smoker | 40.9 (2424) | 40.1 (2377) | 0.02 | 0.763 |
| Peripheral arterial disease | 14.5 (857) | 13.8 (819) | 0.02 | 0.404 |
| Chronic lung disease (moderate/severe) | 28.6 (1693) | 28.8 (1706) | 0.00 | 0.826 |
| Prior myocardial infarction | 45.4 (2692) | 44.8 (2655) | 0.01 | 0.606 |
| Heart failure | 30.9 (1831) | 31.6 (1870) | 0.01 | 0.285 |
| Ejection fraction (%) | 55.0 (50.0-60.0) | 55 (49.0-60.0) | 0.01 | 0.674 |
| Preoperative beta-blocker | 86.7 (5135) | 86.4 (5120) | 0.01 | 0.601 |
| Prior valve surgery | 0.88 (52) | 1.11 (66) | 0.02 | 0.827 |
| Prior CABG | 2.06 (122) | 2.02 (120) | 0.00 | 0.416 |
| Prior cardiac surgery | 3.07 (182) | 3.27 (194) | 0.01 | 0.460 |
| Urgent or emergent status | 52.3 (2696) | 51.4 (2652) | 0.00 | 0.386 |
| Intra-aortic balloon pump (IABP) | 7.21 (427) | 7.39 (438) | 0.00 | 0.229 |
| Predicted risk of mortality | 1.32 (0.75-2.42) | 1.29 (0.76-2.40) | 0.00 | 0.505 |
| Operative characteristics | POAF (n = 5926) | No POAF (n = 5926) | SMD | P-value |
| Procedure | 0.03 | 0.915 | ||
| CABG | 75.5 (4472) | 75.5 (4475) | ||
| AVR | 13.9 (826) | 14.4 (851) | ||
| CABG/AVR | 10.6 (628) | 10.1 (600) | ||
| Number of diseased vessels | 0.00 | 0.958 | ||
| Zero | 10.6 (630) | 11.2 (663) | ||
| One | 6.72 (398) | 6.53 (387) | ||
| Two | 18.1 (1075) | 17.8 (1053) | ||
| Three | 64.5 (3823) | 64.5 (3823) | ||
| Cross clamp time (min) | 71.0 (51.0-92.0) | 71.0 (52.0-92.0) | 0.01 | 0.753 |
| Cardiopulmonary bypass time (min) | 100.0 (78.0-126.0) | 98.0 (77.0-124.0) | 0.02 | 0.145 |
Risk-adjusted outcomes and resource utilization
After risk adjustment, there were no significant differences between groups for postoperative morbidity or mortality [Table 2]. However, postoperative atrial fibrillation continued to be associated with increased resource utilization, including transfusion, increased postoperative length of stay, ICU length of stay, discharge to facilities, and readmission.
Outcomes by POAF status of the match cohort
| Characteristics | POAF (n = 5926) | No POAF (n = 5926) | P-value |
| STS operative mortality | 2.21 (131) | 2.35 (139) | 0.622 |
| STS major morbidity | 10.9 (646) | 11.1 (657) | 0.747 |
| Permanent stroke | 1.27 (75) | 1.47 (87) | 0.343 |
| Prolonged ventilation | 5.87 (348) | 5.97 (354) | 0.815 |
| Renal failure | 2.95 (175) | 2.38 (141) | 0.053 |
| Deep sternal wound infection | 0.30 (18) | 0.42 (25) | 0.285 |
| Reoperation, any cause | 3.14 (186) | 3.27 (194) | 0.677 |
| Transfusion, any | 31.8 (1887) | 26.5 (1573) | < 0.001 |
| Postoperative LOS (days) | 7.0 (5.0-9.0) | 5.0 (4.0-7.0) | < 0.001 |
| ICU LOS (h) | 57.6 (29.0-104) | 48.5 (27.2-80.0) | < 0.001 |
| Discharge to facility | 27.2 (1611) | 23.0 (1362) | < 0.001 |
| Readmission | 11.3 (633) | 8.54 (477) | < 0.001 |
| Total hospital cost | 41,433 (32,802-54,678) | 37,457 (30,578-48,169) | < 0.001 |
| Total stay cost | 12,052 (8347-17,949) | 9889 (6968-14,474) | < 0.001 |
| Diagnostic cost | 3167 (2100-5029) | 2822 (1949-4284) | < 0.001 |
| Intervention cost | 17,893 (14,231-24,360) | 17,566 (13,882-23,525) | < 0.001 |
| General care cost | 6048 (4046-9161) | 5123 (3471-7689) | < 0.001 |
| Other care cost | 0 (0-330) | 0 (0-283) | 0.200 |
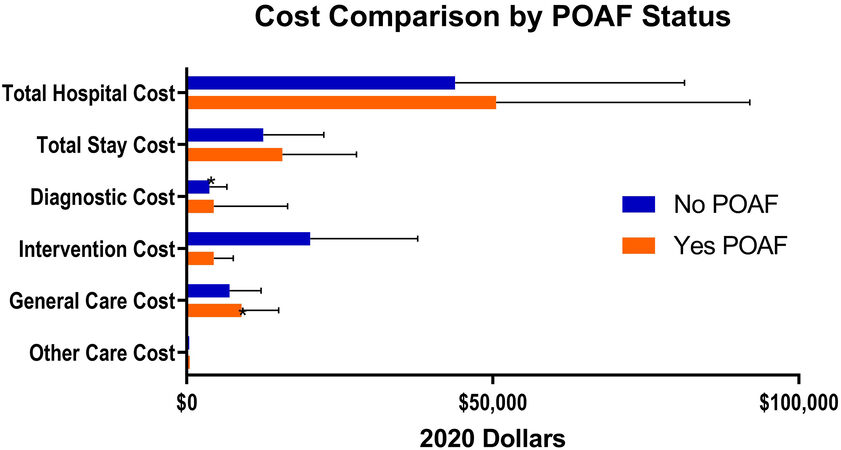
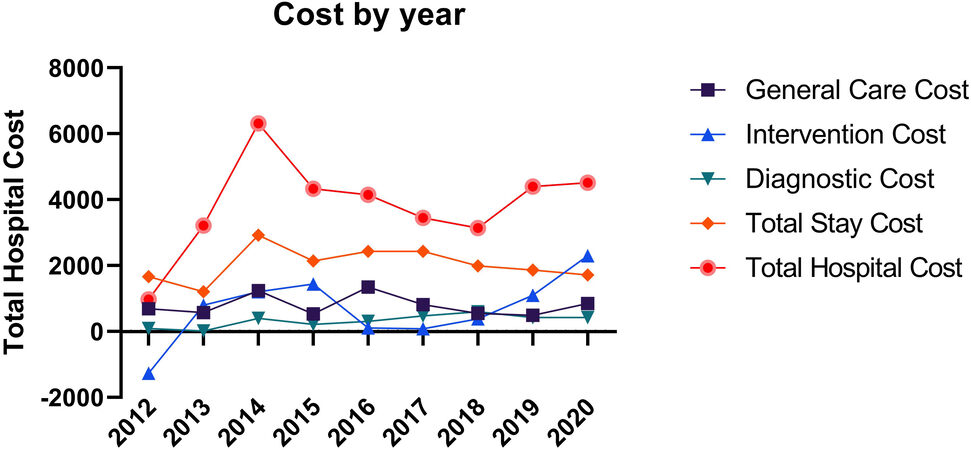
The unpaired cost comparisons are seen in [Table 2], with visual representation of mean total and sub-group costs in [Figure 1]. On paired univariate analysis the mean additional cost (95% confidence interval) associated with postoperative atrial fibrillation was $6705 (5568-7842) in total hospital cost, $3159 (2720-3598) for total stay cost, $699 (545-854) for diagnostic cost, $799 (391-1207) for intervention cost, $1971 (1599-2342) for general care cost, and $77 (2-152) for other costs. All paired differences were statistically significant at P < 0.0001 except for other cost (P = 0.047). Cost estimates over times did not demonstrate either an upward or downward trend [Figure 2].
Figure 1. Cost subgroups by postoperative atrial fibrillation status showing mean ± standard deviation in 2020 dollars.
Sensitivity analysis: patients without post-operative major morbidity or mortality
A sensitivity analysis was undertaken among patients who did not experience postoperative major morbidity or mortality. 5169 pairs of patients were included in the sensitivity analysis. Similar before, these pairs were well matched with all baseline covariates having a standardized mean difference of < 10% [Table 3]. Median predicted risk of mortality was similar between both groups of matched patients (1.32% vs. 1.29%, P = 0.985).
Baseline and operative characteristics by POAF status of the matched subgroup of patients not experiencing major morbidity or mortality
| POAF (n = 5159) | No POAF (n = 5159) | SMD | P-value | |
| Age | 69.0 (63.0-75.0) | 69.0 (63.0-75.0) | 0.01 | 0.716 |
| BSA | 2.09 (1.92-2.27) | 2.08 (1.92-2.26) | 0.01 | 0.449 |
| Female | 24.3 (1252) | 23.7 (1225) | 0.01 | 0.534 |
| Hypertension | 88.5 (4568) | 88.4 (4562) | 0.00 | 0.853 |
| Diabetes | 43.0 (2219) | 43.3 (2233) | 0.01 | 0.781 |
| Dialysis dependent renal failure | 2.36 (122) | 2.73 (141) | 0.02 | 0.235 |
| Prior stroke | 7.97 (411) | 7.46 (385) | 0.02 | 0.337 |
| Smoker | 39.3 (2027) | 39.6 (2042) | 0.01 | 0.762 |
| Peripheral arterial disease | 13.8 (712) | 13.2 (683) | 0.02 | 0.404 |
| Chronic lung disease (moderate/severe) | 27.7 (1431) | 27.5 (1421) | 0.00 | 0.826 |
| Prior myocardial infarction | 44.1 (2274) | 43.6 (2248) | 0.01 | 0.606 |
| Heart failure | 29.9 (1491) | 28.0 (1442) | 0.02 | 0.285 |
| Ejection fraction (%) | 55.0 (50.0-60.0) | 55.0 (50.0-60.0) | 0.01 | 0.674 |
| Preoperative beta-blocker | 86.7 (4466) | 86.9 (4484) | 0.01 | 0.601 |
| Prior valve surgery | 0.85 (44) | 0.81 (42) | 0.00 | 0.828 |
| Prior CABG | 2.04 (105) | 2.27 (117) | 0.02 | 0.416 |
| Prior cardiac surgery | 2.97 (153) | 3.22 (166) | 0.01 | 0.460 |
| Urgent or emergent status | 52.3 (2696) | 51.4 (2652) | 0.02 | 0.386 |
| Intra-aortic balloon pump (IABP) | 5.41 (279) | 4.88 (252) | 0.02 | 0.229 |
| Predicted risk of mortality | 1.32 (0.75-2.42) | 1.29 (0.76-2.39) | 0.00 | 0.985 |
| Operative characteristics | POAF (n = 5159) | No POAF (n = 5159) | SMD | P-value |
| Procedure | 0.00 | 0.915 | ||
| CABG | 75.3 (3885) | 75.5 (3896) | ||
| AVR | 14.3 (740) | 14.4 (742) | ||
| CABG/AVR | 10.4 (534) | 10.1 (521) | ||
| Number of diseased vessels | 0.00 | 0.958 | ||
| Zero | 11.1 (571) | 11.0 (566) | ||
| One | 6.86 (354) | 6.65 (343) | ||
| Two | 18.6 (961) | 18.9 (976) | ||
| Three | 63.4 (3273) | 63.5 (3274) | ||
| Cross clamp time (min) | 71.0 (51.0-91.0) | 70.0 (52.0-92.0) | 0.01 | 0.753 |
| Cardiopulmonary bypass time (min) | 99.0 (78.0-125) | 98.0 (77.0-123) | 0.01 | 0.145 |
The unpaired cost comparisons are seen in [Table 4]. On paired univariate analysis the mean additional cost (95% confidence interval) associated with postoperative atrial fibrillation was $4407 (3690-5123) in total hospital cost, $2357 (2052-2662) for total stay cost, $412 (310-514) for diagnostic cost, $603 (278-929) for intervention cost, $1063 (843-1284) for general care cost, and $-29 (-100-42) for other costs. All paired differences were statistically significant at P < 0.001 except for other cost (P = 0.427).
Outcomes by POAF status of the matched subgroup of patients not experiencing major morbidity or mortality
| Characteristics | POAF (n = 5159) | No POAF (n = 5159) | P-value |
| STS operative mortality | 0 | 0 | N/A |
| STS major morbidity | 0 | 0 | N/A |
| Permanent stroke | 0 | 0 | N/A |
| Prolonged ventilation | 0 | 0 | N/A |
| Renal failure | 0 | 0 | N/A |
| Deep sternal wound infection | 0 | 0 | N/A |
| Reoperation, any cause | 0 | 0 | N/A |
| Transfusion, any | 26.4 (1362) | 22.4 (1153) | < 0.001 |
| Postoperative LOS (days) | 7.0 (5.0-9.0) | 5.0 (4.0-7.0) | < 0.001 |
| ICU LOS (h) | 50.5 (27.5-94.4) | 47.1 (26.4-72.8) | < 0.001 |
| Discharge to facility | 24.2 (1249) | 21.2 (1092) | < 0.001 |
| Readmission | 10.8 (539) | 7.49 (372) | < 0.001 |
| Total hospital cost | 39,598 (31,960-50,362) | 36,142 (29,773-44,526) | < 0.001 |
| Total stay cost | 11,384 (8073-16,130) | 9429 (6720-13,273) | < 0.001 |
| Diagnostic cost | 2968 (2019-4487) | 2677 (1869-3962) | < 0.001 |
| Intervention cost | 17,352 (13,978-23,244) | 16,942 (13,720-22,207) | < 0.001 |
| General care cost | 5633 (3855-8013) | 4893 (3298-6932) | < 0.001 |
| Other care cost | 0 (0-232) | 0 (0-204) | 0.857 |
DISCUSSION
This large multi-institutional study from the Southeastern United States demonstrated significant baseline differences between patients with vs. without POAF consistent with known risk factors [Supplementary Table 2]. After matching, there was a risk-adjusted association between POAF and increased resource utilization, including length of stay, hospital costs, discharge to facilities, and readmission. Although risk adjustment can be difficult in this population, patients were matched on preoperative, intraoperative, and postoperative complications, thereby providing conservative estimates for the impact of POAF. The additional total hospital cost attributable to postoperative atrial fibrillation was a mean of $6705 by paired analysis, and $4407 in a sensitivity analysis. Component costs of hospital stay, diagnostics, intervention, and general care were all similarly increased in patients with POAF. The overall postoperative length of stay was 2 days, and ICU length of stay was 9 h longer for POAF patients. Finally, patients with POAF were 18% more likely to be discharged to a facility, and 33% more likely to be readmitted.
Prior work from our group has identified the incremental costs associated with certain complications, and the accumulation of multiple major morbidities increases costs exponentially[10,11]. Furthermore, patients who develop a single complication are at increased risk for subsequent complications, and these additional costs should be modeled on a logarithmic order. While the STS risk predictor tool does an outstanding job predicting many clinical outcomes, the society does not offer a POAF risk predictor because of poor model performance. Additionally, the existing STS models do accurately risk-adjust for cost[12-14]. It is extremely rare for studies evaluating POAF to adjust for postoperative complications, yet it is critical to do so as POAF and other major morbidities are correlated due to increases in the underlying etiologies of POAF[1,6]. Therefore, this study cannot assess the impact of POAF on postoperative complications, although it does isolate the impact of POAF on resource utilization.
Because of this extensive risk adjustment, the estimates provided in this study are conservative, and fall at the low end of published literature. For example, an additional 9 h in the ICU and 2 days overall, falls at the low end of estimates, which range from 12-48 h of ICU time and 2-5 overall days[1,15-18]. Approximately 13% of readmissions are attributable to atrial fibrillation, yet few studies analyze this outcome in a risk-adjusted manner[19]. In this study, we found a 33% increase in readmissions, which actually mirrors the unadjusted estimates[15]. Finally, our POAF associated cost estimates fall at the low end, which typically ranges from $10,000-$20,000[1]. Total costs attributable to POAF were between $4407 (the mean by paired analysis in patients without major complications) and $6705 (mean of paired analysis among all matched pairs). There can be widely different courses of postoperative atrial fibrillation, and this variability makes simple and precise estimates of resource utilization difficult. This can be seen in the wide confidence intervals of [Figure 1], and the variability in cost estimates over time in [Figure 2]. Although the results are semi-imprecise estimates, the impact is consistent over time and broad, impacting all subgrouping of cost and all stages of postoperative care (ICU, acute care, and outpatient).
In the current environment of quality improvement and focus on protocol-driven care, it is critical to evaluate and understand the implications of postoperative complications as well as targeted approaches to reduce these events. Prophylactic amiodarone is one such intervention that has been demonstrated to reduce the rate of POAF[20-23]. Our group has previously evaluated the cost-effectiveness of this intervention demonstrating prophylactic amiodarone was cost-effective with a savings of $458 per patient treated[11]. While there are many pitfalls in designing, implementing, and evaluating the success of targeted interventions, it is critical to continuously analyze the outcome to ensure continued effectiveness[24]. Cardiac surgery programs may be inundated with various protocols and initiatives to improve the overall care of patients. However, using the value framework, these initiatives should be evaluated on the basis of cost-effectiveness using specific cost estimates that have been derived in this study. The persistence of POAF and stability of cost implications over time shows the difficulty in implementing protocols to reduce its impact on clinical outcomes and resource utilization.
Given the current crisis in healthcare costs and lack of transparency in pricing, it is critical to define resource utilization. In order to more appropriately account for these healthcare-related expenses, it is paramount to understand and quantify the impact of adverse events after cardiac surgery. A recent paper from our group investigated the effects of bundled payments for CABG and identified postoperative complications, including POAF, to be a major driver of cost variability[14]. Any moves toward bundled payment will include postoperative care, typically up to 90 days. Our finding of an 18% increase in discharges to a facility significantly increases overall resource utilization, and in a way that hospitals and practices may be responsible for in the future. Additionally, the COVID-19 pandemic has stressed our health systems to the breaking point, highlighting the importance of resources, including bed allocation, critical care resources, and healthcare providers. By better understanding and predicting the complex healthcare needs of cardiac surgery patients, we can allocate appropriate resources and develop health system-wide strategic plans.
The limitations of this study include its retrospective nature with the risk of selection bias and the inability to determine causality. Risk adjustment was performed using propensity matching, although this does not account for unmeasured confounders. One important unmeasured factor is perioperative hemodynamics, which is unfortunately not available within the regional STS database. This missing information limits a specific analysis of how POAF increases the length of stay, whether that is from hemodynamic instability, anti-arrhythmic or anticoagulation initiation, or other unmeasured aspects of care. While we have data on discharge medications, we do not know the conversion rate and the number of patients discharged in sinus rhythm, although this can be expected to be 95%[17]. Data incompleteness is another inherent limitation of database studies, and the number of patients identified with preoperative atrial fibrillation may be a small underestimate at 3.6%[17]. Finally, only short-term outcomes could be analyzed due to the limited data available in all STS-related databases.
In conclusion, in this regional analysis of the Southeastern United States, postoperative atrial fibrillation was associated with between $4407 and $6705 in total hospital costs after adjusting for baseline risk and other postoperative complications. Nearly all component costs were similarly higher for patients with POAF. The additional $3159 in total stay costs were driven by an increased length of stay of 2 days overall, and 9 h in the ICU. The increase in resource utilization extends beyond the index hospitalization, including increased discharges to a facility and a higher number of readmissions. These results reinforce the continued monetary and clinical impacts of postoperative atrial fibrillation on cardiac surgery patients and providers.
DECLARATIONS
Authors’ contributionsDesign of the study: Hawkins RB, Strobel RJ, Joseph M, Quader M, Teman NR, Almassi GH, Mehaffey JH
Acquisition of data: Hawkins RB
Analysis and interpretation of data: Hawkins RB
Drafting of manuscript: Hawkins RB
Critical revisions: Hawkins RB, Strobel RJ, Joseph M, Quader M, Teman NR, Almassi GH, Mehaffey JH
Approval of manuscript: Hawkins RB, Strobel RJ, Joseph M, Quader M, Teman NR, Almassi GH, Mehaffey JH
Availability of data and materialsNot available.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg 2017;52:665-72.
2. Melby SJ, George JF, Picone DJ, et al. A time-related parametric risk factor analysis for postoperative atrial fibrillation after heart surgery. J Thorac Cardiovasc Surg 2015;149:886-92.
3. Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace 2012;14:159-74.
4. Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol 2019;16:417-36.
5. Mehaffey JH, Hawkins RB, Byler M, et al. Virginia Cardiac Services Quality Initiative. Cost of individual complications following coronary artery bypass grafting. J Thorac Cardiovasc Surg 2018;155:875-82.e1.
6. Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 2008;51:793-801.
7. Services CfMaM. Market Basket Data. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/MarketBasketData.html [Last accessed on 29 Mar 2022].
8. Surgeons SoT. Adult Cardiac Surgery Data Collection. Available from: http://www.sts.org/sts-national-database/database-managers/adult-cardiac-surgery-database/data-collection#data2017 [Last accessed on 29 Mar 2022].
9. Shahian DM, O’Brien SM, Filardo G, et al. Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann Thorac Surg 2009;88:S2-22.
10. Speir AM, Kasirajan V, Barnett SD, Fonner E Jr. Additive costs of postoperative complications for isolated coronary artery bypass grafting patients in Virginia. Ann Thorac Surg 2009;88:40-5; discussion 45-6.
11. Mehaffey JH, Hawkins RB, Byler M, et al. Amiodarone protocol provides cost-effective reduction in postoperative atrial fibrillation. Ann Thorac Surg 2018;105:1697-702.
12. LaPar DJ, Stukenborg GJ, Guyer RA, et al. Primary payer status is associated with mortality and resource utilization for coronary artery bypass grafting. Circulation 2012;126:S132-9.
13. Yount KW, Isbell JM, Lichtendahl C, et al. Bundled payments in cardiac surgery: is risk adjustment sufficient to make it feasible? Ann Thorac Surg 2015;100:1646-52; discussion 1652.
14. Hawkins RB, Mehaffey JH, Yount KW, et al. Investigators for the Virginia Cardiac Services Quality Initiative. Coronary artery bypass grafting bundled payment proposal will have significant financial impact on hospitals. J Thorac Cardiovasc Surg 2018;155:182-8.
15. LaPar DJ, Speir AM, Crosby IK, et al. Investigators for the Virginia Cardiac Surgery Quality Initiative. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg 2014;98:527-33; discussion 533.
16. Almassi GH, Wagner TH, Carr B, et al. VA #517 Randomized On/Off Bypass (ROOBY) Study Group. Postoperative atrial fibrillation impacts on costs and one-year clinical outcomes: the Veterans Affairs Randomized On/Off Bypass Trial. Ann Thorac Surg 2015;99:109-14.
17. Almassi GH, Pecsi SA, Collins JF, Shroyer AL, Zenati MA, Grover FL. Predictors and impact of postoperative atrial fibrillation on patients’ outcomes: a report from the Randomized On Versus Off Bypass trial. J Thorac Cardiovasc Surg 2012;143:93-102.
18. Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg 1997;226:501-11; discussion 511-3.
19. D’agostino RS, Jacobson J, Clarkson M, Svensson LG, Williamson C, Shahian DM. Readmission after cardiac operations: prevalence, patterns, and predisposing factors. J Thorac Cardiovasc Surg 1999;118:823-32.
20. Butler J, Harriss DR, Sinclair M, Westaby S. Amiodarone prophylaxis for tachycardias after coronary artery surgery: a randomised, double blind, placebo controlled trial. Br Heart J 1993;70:56-60.
21. Hoffmann MC. Evaluation of an evidence-based practice implementation: prophylactic amiodarone following coronary artery revascularization. Dimens Crit Care Nurs 2012;31:193-201.
22. Rostagno C. Recent developments in pharmacologic prophylaxis of atrial fibrillation in patients undergoing surgical revascularization. Cardiovasc Hematol Agents Med Chem 2009;7:137-46.
23. Chatterjee S, Sardar P, Mukherjee D, Lichstein E, Aikat S. Timing and route of amiodarone for prevention of postoperative atrial fibrillation after cardiac surgery: a network regression meta-analysis. Pacing Clin Electrophysiol 2013;36:1017-23.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Hawkins RB, Strobel RJ, Joseph M, Quader M, Teman NR, Almassi GH, Mehaffey JH. Postoperative atrial fibrillation is associated with increased resource utilization after cardiac surgery: a regional analysis of the Southeastern United States. Vessel Plus 2022;6:42. http://dx.doi.org/10.20517/2574-1209.2021.116
AMA Style
Hawkins RB, Strobel RJ, Joseph M, Quader M, Teman NR, Almassi GH, Mehaffey JH. Postoperative atrial fibrillation is associated with increased resource utilization after cardiac surgery: a regional analysis of the Southeastern United States. Vessel Plus. 2022; 6: 42. http://dx.doi.org/10.20517/2574-1209.2021.116
Chicago/Turabian Style
Hawkins, Robert B., Raymond J. Strobel, Mark Joseph, Mohammed Quader, Nicholas R. Teman, G. Hossein Almassi, J. Hunter Mehaffey. 2022. "Postoperative atrial fibrillation is associated with increased resource utilization after cardiac surgery: a regional analysis of the Southeastern United States" Vessel Plus. 6: 42. http://dx.doi.org/10.20517/2574-1209.2021.116
ACS Style
Hawkins, RB.; Strobel RJ.; Joseph M.; Quader M.; Teman NR.; Almassi GH.; Mehaffey JH. Postoperative atrial fibrillation is associated with increased resource utilization after cardiac surgery: a regional analysis of the Southeastern United States. Vessel Plus. 2022, 6, 42. http://dx.doi.org/10.20517/2574-1209.2021.116
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 8 clicks
Cite This Article 8 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.