Grey zones in the supportive treatments of cardiac amyloidosis
Abstract
Recent advances in the diagnosis and treatment of cardiac amyloidosis (CA) have translated into a longer life expectancy of patients and more challenging clinical scenarios. Compared to the past, patients with CA and heart failure (HF) currently encountered in clinical practice are a more heterogeneous population and require tailored strategies. The perception of CA as a treatable disease has opened new possibilities for the management of these patients, but many grey areas remain to be explored. The aim of this review is to provide practical suggestions for daily clinical activity in the management of challenging scenarios in CA, including the effectiveness and tolerability of evidence-based HF medication; rate vs. rhythm control in atrial fibrillation, thromboembolic risk, and anticoagulation therapies; replacement of severe aortic valve stenosis; the impact of implantable cardioverter defibrillator on survival; and the usefulness of cardiac resynchronization therapy.
Keywords
INTRODUCTION
Cardiac amyloidosis (CA) is characterized by extracellular deposition of misfolded proteins, mostly immunoglobulin light chains (AL) produced by an abnormal clonal proliferation of bone marrow plasma cells and transthyretin (TTR) protein. In TTR amyloidosis (ATTR), the mechanism leading to tissue infiltration are related to age-related failure of homoeostatic mechanisms in wild-type TTR (wtTTR) or destabilizing mutations in variant TTR (vTTR)[1]. The epidemiology of CA is rapidly evolving due to increased awareness of disease, identification of clinical red flags, and the possibility to achieve a non-invasive diagnosis with cardiac scintigraphy with bone tracers[2,3].
Furthermore, the availability of novel therapeutic strategies both in AL and ATTR-CA with proven impact on survival has provided a new impulse towards an early diagnosis[4,5]: bortezomib and daratumumab for AL amyloidosis[6,7] and TTR stabilizers, small interfering RNA, and antisense oligonucleotides for ATTR amyloidosis[4]. While chemotherapy has long been studied for the treatment of AL-CA and can improve overall outcome, data of a survival benefit from specific treatments for ATTR-CA currently come from a single clinical trial[8]. Therefore, a multidisciplinary approach is essential for the management of these patients[9]. CA is currently recognized as a relatively prevalent, still under-diagnosed disease and an emerging cause of heart failure (HF) and mortality[10]. In particular, specific populations have been reported at increased prevalence of ATTR-CA[10]: 13% of patients with HF with preserved ejection fraction (HFpEF)[11], 5% of patients with a previous diagnosis of hypertrophic cardiomyopathy, 10% of patients with unexplained cardiac hypertrophy at the time of carpal tunnel syndrome[12], and 16% of those with severe calcific aortic valve stenosis (AS) undergoing transcatheter aortic valve implantation (TAVI)[13,14] [Table 1].
Knowledge and uncertainty in the management of cardiac amyloidosis
| Knowledge | Uncertainties | ||
| AF | Rate control | Improves HF symptoms; Drugs commonly used can be poorly tolerated and have a narrow therapeutic window; Low-dose BB is generally safe and well-tolerated; Digoxin should be avoided; AV node ablation with biventricular PM implantation might be an option when other strategies fail | What is the prognostic impact of rate control compared to rhythm control? Is rate control the strategy of choice? Which drugs should be used? When is rate preferred to rhythm control? |
| Rhythm control | Exclude cardiac thrombosis before cardioversion; AF recurrence is frequent; Amiodarone is the agent of choice. Ibutilide and propafenone might be used under close monitoring of the QT interval and renal function; DCCV has significant risk of complications; Catheter ablation might be an option, but data are scarce and controversial | Does it improve HF symptoms? Does maintaining SR have a prognostic impact? Are markers of atrial remodeling commonly used in HF reliable for predicting the risk of AF recurrence after cardioversion? When should catheter ablation be performed? Should PV isolation be associated with other ablation targets? | |
| Thromboembolism & Anticoagulation | In AF | Anticoagulation is recommended regardless of the CHA2DS2-VASc score; Bleeding risk is frequently increased in CA (especially AL) and should be carefully assessed before initiation of anticoagulation | Which is the first-line anticoagulant in CA without other indications? Are DOACs as effective as warfarin? Should LAA closure be considered in patients with prohibitive bleeding risks? |
| In SR | Advanced atrial dysfunction promotes embolic events; CHA2DS2-VASc score ≥ 3 is associated with increased thromboembolic risk | Should anticoagulation be used in SR? Should cardiac thrombosis be excluded in all patients? How should we identify the best candidates? Are transmitral A wave < 20 cm/s or LAA velocities < 40 cm/s useful? | |
| CAD | CAD should be regularly assessed and promoting factors should be treated as in patients without CA; Treatment of significant stenosis of the main epicardial coronaries confers a more benign outcome; Low cardiac output syndrome is frequent after coronary artery bypass surgery; Coronary revascularization might not ameliorate symptoms due to microvascular dysfunction | Should PCI be preferred to surgery? How shoulw we manage anti-thrombotic therapy? How can we predict the risk of low cardiac syndrome after CABG? How can we predict the impact of revascularization on symptoms and prognosis? How can microvascular dysfunction be relieved? | |
| Treatment of AS | Accurate characterization of AS is fundamental; Untreated severe AS confers worse prognosis; The prognostic benefit of treating LF-LG AS is debated; TAVI seems to improve the outcome of ATTR-CA patients compared to medical management; LVEF to monitor disease progression is an inaccurate parameter in heavily hypertrophied hearts | When should we treat AS? Should LF-LG AV be regularly treated? How should we monitor disease progression? How can we predict the feasibility of TAVI and the risk of complications? Should dual antiplatelet therapy follow TAVI? Should patients with CAD and AS be treated with PCI and TAVI or CABG and AVR? | |
| SCD and ICD | Ventricular arrhythmias are common in CA; No survival benefit is demonstrated following ICD implantation in CA; AL-CA exhibits a high frequency of appropriate ICD shocks; ICD implantation for secondary prevention can be considered in AL or hereditary ATTR-CA (Class IIa, level of evidence C); The role of prophylactic ICD remains controversial, but it might be considered as bridge to transplant or in ischemic heart disease fulfilling indications for implantation | Which parameters should guide the decision to implant an ICD? Could patients with biventricular systolic dysfunction benefit from ICD placement? How can we predict the risk of SCD with shockable rhythm to guide prophylactic ICD implantation? How should we select patients for secondary prevention of SCD? Do ATTR and AL deserve different strategies for SCD prevention? | |
| CRT | LBBB is frequent in ATTR-CA at diagnosis; Decline in LVEF is a late phenomenon in CA; There are no defined indications to CRT in CA; PM-dependent patients and those with frequent RV pacing (> 40% of the time) might benefit from CRT implantation. | Is there a prognostic benefit of CRT in CA? Is CRT effective in CA patients fulfilling indications according to latest HF guidelines? Should CRT be offered based on HF symptoms and typical LBBB, irrespective of LVEF? Might para-hissian pacing be feasible in CA? Should it be preferred to RV pacing? |
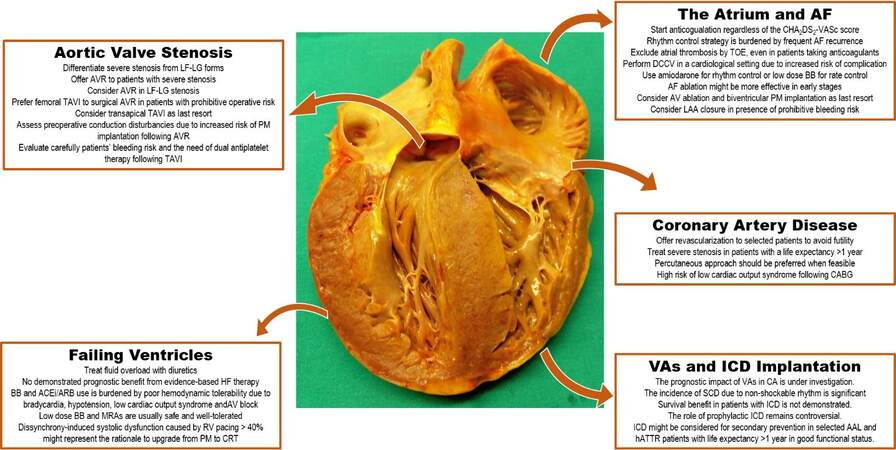
The epidemiology of the disease, the contemporary natural history of CA, and the identification of tools for accurate prognostic stratification are the fundamental questions awaiting clarification. In this evolving scenario, the optimal “supportive” treatments for CA patients with HF represents a largely unexplored field[15]. This review focuses on the effectiveness and tolerability of evidence-based HF medication, rate vs. rhythm control in atrial fibrillation (AF), thromboembolic risk and anticoagulation therapies, replacement of severe AS, impact of implantable cardioverter defibrillator (ICD) on survival, and usefulness of cardiac resynchronization therapy (CRT) [Figure 1]. The aim is to provide practical suggestions for daily clinical activity in the management of patients with CA.
Figure 1. Current management and grey areas in cardiac amyloidosis. Courtesy of Professor Rossana Bussani, MD, Institute of Pathological Anatomy and Histology, University of Trieste, Italy. AL: Light chain amyloidosis; ACE-i/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; AF: atrial fibrillation; AV: atrio-ventricular; AVR: aortic valve replacement; BBs: beta blockers; CA: cardiac amyloidosis, CRT: cardiac resynchronization therapy; DCCV: direct current cardioversion; hATTR: hereditary transthyretin amyloidosis; HF: heart failure; ICD: implantable cardioverter defibrillator; LAA: left atrial appendage; LF-LG: low-flow low-gradient; LVEF: left ventricular ejection fraction; MRAs: mineral receptor antagonists; PM: pacemaker; RV: right ventricle; SCD: sudden cardiac death; SR: sinus rhythm; TAVI: transcatheter aortic valve implantation; TOE: transesophageal echocardiography; VAs: ventricular arrhythmias.
Pathophysiology and mechanisms of cardiac damage in AL and TTR CA
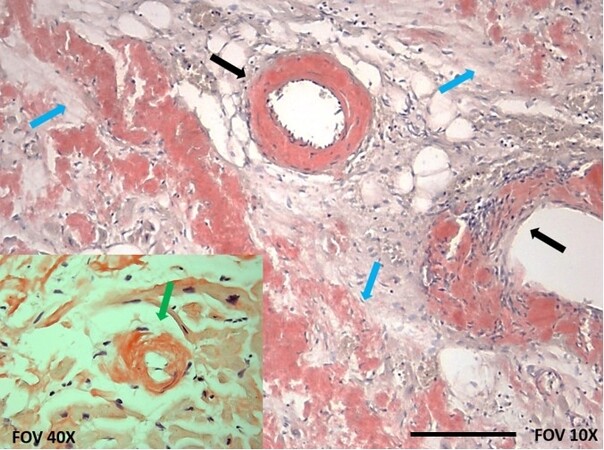
While AL-CA results from a plasma cell dyscrasia where clonal plasma cells produce monoclonal amyloid precursors, ATTR-CA derives from the cardiac infiltration by wtTTR or vTTR proteins produced mostly by the liver[2]. Although the atria and ventricles are typically involved, amyloid can infiltrate every area of the heart[16] [Figure 2]. Cardiac damage results from a double-hit process involving amyloid deposition and a direct “toxic” effect exerted by circulating preamyloid proteins causing oxidative stress and mitochondrial damage, especially for free light chains[17-19]. TTR oligomers and protofibrils have been demonstrated to induce nerve cytotoxicity by caspase-3 activation, triggering the onset of programmed cell death[20]. Although exposure of cardiomyocytes to TTR protofilaments is thought to be mediated by a similar mechanism, conclusive evidence of a direct cardiac damage is currently lacking. Thickened biventricular walls, dilatation of both atria, and poor diastolic filling due to noncompliant ventricles are hallmarks of CA[16]. The increase in myocardial mass determines a progressively smaller ventricular cavity size, resulting in a condition of fixed end-diastolic volume (i.e., pre-load)[21]. In this model of cardiomyopathy, right and left ventricular ejection fraction (LVEF) tend to be preserved up until higher burdens of cardiac infiltration. In the presence of overt restrictive pathophysiology, severe dilatation of both atria occurs, cardiac output becomes critically dependent on heart rate, and arterial hypotension is established, reflecting the most advanced stages of the disease[22].
Figure 2. Atrial amyloidosis. Courtesy of Professor Rossana Bussani, MD, Institute of Pathological Anatomy and Histology, University of Trieste, Italy. Congo Red staining revealed amyloid infiltration extending to interstitial space (blue arrows) and vessel walls (black arrows) including the sinoatrial nodal artery. A focus at 40× (bottom left) shows a cardiomyocyte (green arrow) surrounded by amyloid deposits. Scale bar, 500 μm.
AL and ATTR amyloidosis: different extracardiac clinical manifestations
Patients with AL amyloidosis frequently present with renal and autonomic nervous system involvement, but gastrointestinal and soft tissue involvement can occur[23]. Most patients have normal serum creatinine levels at diagnosis and progressive renal failure develops in ≈20% of cases. Proteinuria results from amyloid deposition at the glomerular level and can evolve to the nephrotic range in 30%-50% of cases, sometimes leading to hypoalbuminemia requiring supplementation. Autonomic neuropathy can present with symptomatic orthostatic hypotension of different magnitude, ranging from only associated with exertion to severe enough to prevent the completion of ordinary activities.
Gastrointestinal involvement might cause weight loss, malabsorption, gastrointestinal bleeding, and development of motility disturbances. Hepatomegaly and increased serum alkaline phosphatase levels suggest liver infiltration.
Extracardiac involvement in wtTTR amyloidosis predominantly consists in the presence of carpal tunnel syndrome (commonly with bilateral manifestations)[24]. Conversely, vTTR amyloidosis frequently manifests as sensorimotor polyneuropathy accompanied by autonomic symptoms[9].
METHODS
This is a narrative review dealing with the most frequent clinical questions and needs encountered in the management of patients with CA in everyday practice. The content of each section addresses a specific topic providing information based on experts’ opinions supported by scientific literature.
GREY ZONES
Neurohormonal antagonists and supportive treatment
Peculiar pathophysiological conditions limit the introduction and up-titration of HF medications in CA. In detail, beta-blockers (BBs) are generally considered contraindicated or poorly tolerated due to unfavorable hemodynamic effects of slowing the heart rate because of inability to adequately increase cardiac output[21]. In addition, CA patients are prone to hypotension and electrical conduction abnormalities, particularly atrio-ventricular (AV) blocks, which can be worsened by BBs[2]. Particularly in AL-CA, orthostatic hypotension is commonly reported due to dysfunction of the autonomic nervous system or toxicity from chemotherapy agents[21]. These medications might be better tolerated in patients with ATTR-CA lacking significant autonomic neuropathy.
Available data about the use of HF medications in CA are controversial and derive from retrospective studies [Table 1]. However, concerns about safety of BBs, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACE-i/ARBs), and mineral receptor antagonists (MRAs) were evident in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) as fewer than 30% of patients were treated with these medications[8]. In recent real-world reports, BBs were frequently prescribed with up to 40% of CA patients under therapy at first evaluation[25] and well-tolerated in the absence of known contraindications[26].
In a retrospective cohort of 99 patients with AL- and ATTR-CA followed for 16 months, BBs, ACE-i, and MRAs were well tolerated in the absence of contraindications with fewer than 10% of patients experiencing adverse effects attributable to these drugs (most frequently, hypotension and symptomatic bradycardia)[27]. Conversely, in a single-center, retrospective study including 480 ATTR-CA patients, the use of BBs and ACE-i reduced survival in variant ATTR-CA and did not affected outcome in wild-type ATTR-CA at 41 months[28]. In this study, patients on HF medications had a more severe cardiac involvement, thus potentially having a worse outcome per se. Therefore, the safety, tolerability, and prognostic impact of anti-neurohormonal drugs need to be tested in further studies, differentiating ATTR- from AL-CA. Meanwhile, drugs for neurohormonal antagonism should be started at a low dose and slowly up-titrated under close monitoring of patients, particularly in AL-CA.
Along with anti-neurohormonal drugs, salt restriction (< 6 g/day) and diuretics are the mainstay of supportive treatment in CA. Fluid overload might result from nephrotic syndrome with hypoalbuminemia or concomitant therapies such as steroids in AL and diflunisal in ATTR. In the case of scarce response to loop diuretics, MRAs are well tolerated[23]. However, over-diuresis may lead to hypotension and acute kidney injury, especially in patients with orthostatic hypotension and renal dysfunction with nephrotic syndrome[29]. Diminishing preload, these drugs may compromise an already fixed stroke volume, leading to low cardiac output[21]. Therefore, caution is required as the search for the optimal volume balance in CA is challenged by a narrow therapeutic window.
Atrial fibrillation: rate control or rhythm control?
AF is the most frequent arrhythmia in CA (45%-70% of patients)[30,31]. It commonly leads to hemodynamic deterioration in CA mainly because of fast and irregular heart rates compromising ventricular filling and cardiac output and, to a lesser extent, the loss of atrial contractility. In advanced atrial infiltration, the atrial contribution to diastolic filling might be irrelevant even in sinus rhythm. The management of AF is challenging because agents for rate and rhythm control may be poorly tolerated, and many patients have atrial thrombi precluding the adoption of rhythm control strategies such as direct-current cardioversion (DCCV) [Table 1][32].
The management of AF in patients with CA can be clinically challenging requiring major decisions such as: (1) rate vs. rhythm control strategy; (2) pharmacological vs. electrical therapy (DCCV or AF ablation); and (3) anticoagulant therapy and estimation of the thromboembolic and bleeding risk.
Rate control
The rate control strategy represents the most common indication to start BBs in patients with CA[26]. Safety and tolerability are acceptable when BBs are prescribed at low doses for lenient control of the heart rate (< 110 bpm)[27]. In addition, they might be indicated for concomitant conditions, such as coronary artery disease. In the latest guidelines from the European Society of Cardiology[33], digoxin is recommended for rate control of AF in patients with LVEF ≥ 40% (Class I, LOE B). However, digoxin use in CA has been traditionally discouraged following in vitro studies reporting a tendency to bind to amyloid fibrils, thus increasing the local drug concentrations and potentially resulting in toxic effects[34]. As a consequence, CA is considered an absolute contraindication to digoxin use due to the possibility of increased risk of toxicity even with “therapeutic digoxin levels”[35]. Nevertheless, the clinical significance of these findings is unclear and digoxin in low doses, adjusted according to serum trough levels, has been used with acceptable tolerability[36]. A recent retrospective analysis of 107 AL patients treated with digoxin observed a relatively low incidence of arrhythmias (11% of cases), almost exclusively in newly diagnosed patients[35]. Ongoing studies to shed light upon digoxin use in CA, whether clinicians decide to use this drug, drug concentrations, and renal function should be closely monitored. Similar to digoxin, non-dihydropyridine Ca blockers (i.e., verapamil) and nifedipine bind amyloid fibrils and should be avoided as they can precipitate or worsen HF, even HFpEF, potentially resulting in advanced AV blocks and cardiogenic shock[21,37,38]. Amiodarone is fairly well tolerated, particularly if administered orally, and is effective in achieving rate control.
Rhythm control
Currently, there is not sufficient evidence to recommend pursuing the rhythm rather than rate control strategy in CA. Biatrial dilatation is common in patients with CA and atrial function can be impaired in the presence of advanced cardiac infiltration resulting in a modest end-diastolic contribution to ventricular filling also in sinus rhythm. This makes rhythm control (i.e., DCCV) a less attractive option due to an increased risk of arrhythmias relapse during follow up. In clinical practice, rhythm control options are mainly considered in patients experiencing deleterious effects from drugs for rate control and in those with significant symptom burden in the presence of AF[30,31]. However, a strategy including transesophageal echocardiography before proceeding with pharmacological or electrical cardioversion could be reasonable due to the increased frequency of atrial thrombosis observed even in patients under anticoagulant therapy (up to 28% in recent series)[39].
Amiodarone is the agent of choice in most cases, but other antiarrhythmic drugs are also useful such as dofetilide and propafenone[21]. For most of them, close monitoring of the QT interval and renal function is needed.
Non-pharmacological options include DCCV, AF ablation, and AV node ablation
Success rates of DCCV are similar in patients with and without CA (approaching 90%-95%)[31,40]. However, CA patients require higher mean energy and multiple attempts to restore sinus rhythm and experience more procedural complications (up to a seven-fold increase) compared to non-CA patients, including ventricular arrhythmias and bradyarrhythmias (sometimes requiring PM implantation)[40]. The rates of recurrent arrhythmia can vary from 61% at 30 days to 48%-80% at 1 year following DCCV, depending on the considered populations and median follow-up time[31,40,41].
There is a paucity of clinical experience and data in the literature about the safety and efficacy of AF ablation in CA. In small retrospective cohorts, including both AL and ATTR patients, the procedure was safe and well tolerated, and it was associated with amelioration of the New York Heart Failure (NYHA) functional class and quality of life[42,43]. However, the outcome of CA patients undergoing AF ablation demands further investigation. The recurrence rates of AF following ablation in patients with ATTR-CA are variable: 25% at 12 months, 40% at 36 months, and 58% at 40 months[31,42,43]. The lower is the ATTR-CA stage[44], the lower is the arrhythmia recurrence rate. In a recent investigation, AF ablation was associated with lower rates of hospitalization for HF or arrhythmias and improved survival over a mean follow up of 39 months[31]. If some prognostic benefit exists in treating AF in CA, it is likely that rhythm control strategies are substantially more effective when performed earlier in disease course[31].
When rate control is not tolerated and rhythm control is ineffective, AV node ablation followed by PM implantation could be an option to relief AF symptoms[42]. Placement of the right ventricular lead in the high septal/para-His regions or biventricular pacing should be preferred to prevent deleterious effects of ventricular dyssynchrony from long-term right ventricular pacing[45,46].
Arterial thromboembolism and anticoagulant therapy
Post-mortem and in vivo studies using transesophageal echocardiography and cardiac magnetic resonance demonstrated an increased prevalence of intracardiac thrombosis and arterial thromboembolism in patients with CA, even in sinus rhythm or in AF under anticoagulation therapy[40,47,48]. According to current recommendations, patients with non-valvular AF and increased CHA2DS2-VASc score have a class I indication to start anticoagulation preferably with direct oral anticoagulants (DOACs) as first-line therapy or with vitamin K antagonists (warfarin)[33]. Although this score is widely used for the prediction of thromboembolic events in AF[49], its accuracy has been recently questioned in CA in light of the high prevalence of left atrial appendage (LAA) thrombosis in patients with low CHA2DS2-VASc score[50]. Therefore, Anticoagulation is indicated in most CA patients with AF, regardless of the CHA2DS2-VASc score, and might also be considered in those in sinus rhythm, especially AL-CA, at increased thromboembolic risk[40,51] revealed by spontaneous echo contrast, transmitral A wave < 20 cm/s, and LAA velocities < 40 cm/s[52] or lower[51]. A CHA2DS2-VASc score ≥ 3 has been associated with an almost three-fold increased risk of thromboembolic events in CA patients in sinus rhythm[48], as previously reported in patients without CA[53]. However, the decision to start empirical anticoagulation and its benefit are largely debated.
There are limited data on the optimal anticoagulant strategy in CA. A recent retrospective study comparing the efficacy of anticoagulant regimens reported no differences in the rate of strokes and transient ischemic attack in patients with ATTR-CA treated with DOACs and warfarin (2.9 vs. 3.9/100 person/years, respectively). The decision to initiate anticoagulant therapy should be based on the global assessment of the risk-benefit ratio in each patient [Table 1]. The selection of the specific anticoagulant regimen cannot be supported by evidence in CA yet. While waiting results from further prospective studies with larger CA cohorts, it is reasonable to consider DOACs as first-line therapy, in line with latest guidelines[33], and warfarin in the case of prosthetic mechanical heart valves, moderate-sever mitral stenosis, or recurrent thrombosis while under DOACs. In the case of prohibitive bleeding risks or contraindications to anticoagulation (i.e., active major bleeding or severe thrombocytopenia < 50 platelets/μL), LAA closure devices may be considered[33], even though they have not been studied in CA yet.
Characterization and management of aortic valve stenosis
AS is a common finding in CA, ranging from 6% to 29% of patients, being particularly prevalent in men > 70-75 years old with ATTR-CA[13,54]. Currently, the clinical management and outcome of patients with CA and AS relies on empirical experience rather than official indications [Table 1]. Data from small retrospective studies[55-57] suggest that outcomes following AVR in CA patients are poorer than those in non-CA patients, particularly in the presence of low flow low gradient (LF-LG) AS[58]. In the absence of peripheral artery disease, trans-femoral TAVI might be the strategy of choice[55] at the cost of increased risk of procedural complications (i.e., complete AV block)[59,60].
A recent prospective study, part of ATTRact-AS (a study investigating the role of occult cardiac amyloid in the elderly with aortic stenosis, NCT03029026), investigated the prevalence and outcome of ATTR-CA with severe AS in patients ≥ 75 years old referred for TAVI. Compared to the others, patients with ATTR-CA (13% of the study cohort) had similar incidence of periprocedural complications and overall mortality at a median follow up of 19 months after TAVI. Furthermore, TAVI significantly improved the outcome of ATTR-CA patients with AS compared to medical management. Notably, unlike previous studies, the male:female ratio and the prevalence LF-LG AS were similar in patients with and without ATTR-CA.
Candidates to AVR should be carefully evaluated to define the severity of AS and the possibility of consistent benefits as amyloid-induced myocardial dysfunction is expected to persist even after AVR. The heart team should consider frailty, life expectancy, procedural risks, and comorbidities. Some factors are associated with unfavorable prognosis and futility: LVEF < 50%, restrictive filling pattern, impaired global longitudinal strain (> -10%), and LF-LG AS[55,56]. Recent results are promising and suggest that TAVI is not a futile procedure in patients with CA, but further confirmation in larger cohorts is needed. Future research is required to identify the best candidates.
Ventricular arrhythmias and usefulness of ICD therapy
Non-sustained ventricular tachycardia (VT) can be found in 27% of patients during routine monitoring[61], but this percentage increases up to 74% in patients with implanted devices[62] and 100% of AL-CA patients during stem cell transplant period[63]. The clinical significance and prognostic implications of ventricular arrhythmias in CA are yet to be determined, but recent studies suggest an association with increased mortality[62,64,65]. Therefore, a history of non-sustained VT has been proposed as a useful finding when considering CA patients for ICD implantation[62].
The latest European Society of Cardiology (ESC)[66] and American Heart Association[67] guidelines acknowledge the absence of sufficient evidence for formal recommendation regarding ICD implantation in primary prevention of SCD and support patient-tailored decision-making in CA. The 2015 ESC guidelines recommend (Class IIa, level of evidence C) considering ICD implantation for secondary prevention in AL or hereditary ATTR-CA with “ventricular arrhythmias causing hemodynamic instability who are expected to survive > 1 year with good functional status”[66]. Although secondary prevention is a strong indication for ICD implantation in patients with HF[68], the role of prophylactic ICD in CA remains controversial. Available data from retrospective small cohorts report a high incidence of appropriate ICD discharge, predominantly in AL-CA patients implanted for secondary prevention[62,69,70]. Based on a retrospective analysis of data from 31 patients with AL and ATTR-CA, Varr et al.[62] from the Stanford Amyloid Center proposed considering ICD implantation for primary prevention in patients with NYHA < IV, life expectancy > 1 year, and history of exertional syncope or documentation of VT (either non-sustained or sustained) on ambulatory Holter monitoring. However, studies to date failed to demonstrate a survival benefit following ICD implantation for primary or secondary prevention[70,71], reasonably due to: (1) frequent arrhythmias not amenable to defibrillation (i.e., pulseless electrical activity) representing the predominant cause of arrhythmic death[71,72]; (2) higher defibrillation thresholds of infiltrated hearts[65]; (3) significant proportion of non-cardiac death[73]; and (4) advanced CA at diagnosis carrying ominous prognosis.
While waiting for trials to yield positive results for SCD prevention in CA, a critical approach to select candidates for ICD implantation should include age, presumed life expectancy, severity of CA, major comorbidities increasing competing risks of non-cardiac death, and coexisting conditions with strong indication for SCD prevention (i.e., documented sustained VT of ventricular fibrillation) [Table 1]. For instance, ICD implantation for primary prevention might have a role in CA patients with Stage I-II, previous myocardial infarction, left ventricle (LV) systolic dysfunction, and significant arrhythmic burden or in those being considered for heart transplantation (HTx) as a “bridge” strategy.
Pacemaker and cardiac resynchronization therapy
The development of LV systolic dysfunction is a late phenomenon in CA, and the majority of patients do not fulfil criteria for CRT implantation[68]. A significant percentage of patient with CA, especially ATTR-CA, have LBBB at diagnosis, amenable of CRT. In addition, in up to 30% of cases[74], CA is associated with conduction abnormalities such as advanced AV block requiring PM implantation with long-term RV pacing. Notably, PM-dependent patients and those with frequent RV pacing (> 40% of the time) might benefit from CRT implantation being at higher risk of further functional decline and development of impaired LVEF with worse HF prognosis[46]. In this population, CRT has been associated with amelioration of the NYHA functional class, LVEF, and reduced mitral regurgitation[46]. The few available data on the prognostic impact of CRT in CA are controversial[46,75,76]. Indications and timing for CRT implantation in patients with CA are currently debated [Table 1]. A potential concern for CRT in CA might rise from the need for higher mean energies to deliver effective therapy due to higher capture thresholds of the infiltrated heart[65]. In particular, whether CRT may be an effective therapy and should be offered as first-line therapy in CA patients requiring frequent RV pacing regardless of the LVEF is unknown. Therefore, based on current evidence, it is reasonable to consider CRT in patients with CA who meet guideline criteria for implantation[68].
CONCLUSION
Recent advances in the diagnosis and treatment of CA have translated into longer life expectancy of patients and more challenging clinical scenarios. Compared to the past, patients with CA and HF currently encountered in clinical practice are a more heterogeneous population and require tailored strategies. The perception of CA as a treatable disease has opened new possibilities for the management of these patients, but many grey areas remain to be explored.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and structure of the review: Porcari A
Editing and drafting: Porcari A, Pagura L, Varrà GG, Longo F, Rossi M, Saro R, Barbisan D, Cittar M
Revision, supervision and final approval: Rapezzi C, Merlo M
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2020;27:217-22.
2. Porcari A, Merlo M, Rapezzi C, Sinagra G. Transthyretin amyloid cardiomyopathy: an uncharted territory awaiting discovery. Eur J Intern Med 2020;82:7-15.
3. Maurer MS, Bokhari S, Damy T, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail 2019;12:e006075.
4. Emdin M, Aimo A, Rapezzi C, et al. Treatment of cardiac transthyretin amyloidosis: an update. Eur Heart J 2019;40:3699-706.
5. Merlo M, Porcari A, Pagura L, et al. A national survey on prevalence of possible echocardiographic red flags of amyloid cardiomyopathy in consecutive patients undergoing routine echocardiography: study design and patients characterization-the first insight from the AC-TIVE Study. Eur J Prev Cardiol 2021;zwab127.
6. Kastritis E, Palladini G, Minnema MC, et al. ANDROMEDA Trial Investigators. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med 2021;385:46-58.
7. Sperry BW, Ikram A, Hachamovitch R, et al. Efficacy of chemotherapy for light-chain amyloidosis in patients presenting with symptomatic heart failure. J Am Coll Cardiol 2016;67:2941-8.
8. Maurer MS, Schwartz JH, Gundapaneni B, et al. ATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007-16.
9. Korosoglou G, Giusca S, André F, et al. Diagnostic work-up of cardiac amyloidosis using cardiovascular imaging: current standards and practical algorithms. Vasc Health Risk Manag 2021;17:661-73.
10. Porcari A, Falco L, Lio V, et al. Cardiac amyloidosis: do not forget to look for it. Eur Heart J Suppl 2020;22:E142-7.
11. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585-94.
12. Porcari A, Pagura L, Longo F, et al. Prognostic significance of unexplained left ventricular hypertrophy in patients undergoing carpal tunnel surgery. ESC Heart Fail 2021; doi: 10.1002/ehf2.13606.
13. Castaño A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017;38:2879-87.
14. Nietlispach F, Webb JG, Ye J, et al. Pathology of transcatheter valve therapy. JACC Cardiovasc Interv 2012;5:582-90.
15. Visser RAB, Gravenor C, Ahmed S, Harky A. Amyloidosis and cardiovascular diseases: a clinical insight. J Card Surg 2021;36:522-9.
16. Porcari A, Bussani R, Merlo M, et al. Incidence and characterization of concealed cardiac amyloidosis among unselected elderly patients undergoing post-mortem examination. Front Cardiovasc Med 2021;8:1680.
17. Brenner DA, Jain M, Pimentel DR, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res 2004;94:1008-10.
18. Koike H, Katsuno M. The ultrastructure of tissue damage by amyloid fibrils. Molecules 2021;26:4611.
19. Koike H, Katsuno M. Transthyretin amyloidosis: update on the clinical spectrum, pathogenesis, and disease-modifying therapies. Neurol Ther 2020;9:317-33.
20. Mendes Sousa M, Cardoso I, Fernandes R, Guimarães A, Saraiva MJ. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy. Am J Pathol 2001;159:1993-2000.
21. Kittleson MM, Maurer MS, Ambardekar AV, et al. American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the american heart association. Circulation 2020;142:e7-e22.
22. Knight DS, Zumbo G, Barcella W, et al. Cardiac structural and functional consequences of amyloid deposition by cardiac magnetic resonance and echocardiography and their prognostic roles. JACC Cardiovasc Imaging 2019;12:823-33.
23. Falk RH, Alexander KM, Liao R, Dorbala S. AL (Light-Chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 2016;68:1323-41.
24. Rapezzi C, Lorenzini M, Longhi S, et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev 2015;20:117-24.
25. Canepa M, Tini G, Musumeci B, et al. Real-world versus trial patients with transthyretin amyloid cardiomyopathy. Eur J Heart Fail 2019;21:1479-81.
26. Tini G, Cappelli F, Biagini E, et al. Current patterns of beta-blocker prescription in cardiac amyloidosis: an Italian nationwide survey. ESC Heart Fail 2021;8:3369-74.
27. Aimo A, Vergaro G, Castiglione V, Rapezzi C, Emdin M. Safety and tolerability of neurohormonal antagonism in cardiac amyloidosis. Eur J Intern Med 2020;80:66-72.
28. Aus dem Siepen F, Hein S, Bauer R, Katus HA, Kristen AV. Standard heart failure medication in cardiac transthyretin amyloidosis: useful or harmful? Amyloid 2017;24:132-3.
29. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation 2017;135:1357-77.
30. Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail 2018;5:772-9.
31. Donnellan E, Wazni OM, Hanna M, et al. Atrial fibrillation in transthyretin cardiac amyloidosis: predictors, prevalence, and efficacy of rhythm control strategies. JACC Clin Electrophysiol 2020;6:1118-27.
32. Feng D, Edwards WD, Oh JK, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007;116:2420-6.
33. Hindricks G, Potpara T, Dagres N, et al. ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498.
34. Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation 1981;63:1285-8.
35. Muchtar E, Gertz MA, Kumar SK, et al. Digoxin use in systemic light-chain (AL) amyloidosis: contra-indicated or cautious use? Amyloid 2018;25:86-92.
36. Cheung CC, Roston TM, Andrade JG, Bennett MT, Davis MK. Arrhythmias in cardiac amyloidosis: challenges in risk stratification and treatment. Can J Cardiol 2020;36:416-23.
37. Gertz MA, Skinner M, Connors LH, Falk RH, Cohen AS, Kyle RA. Selective binding of nifedipine to amylold fibrils. Am J Cardiol 1985;55:1646.
38. Gertz MA, Falk RH, Skinner M, Cohen AS, Kyle RA. Worsening of congestive heart failure in amyloid heart disease treated by calcium channel-blocking agents. Am J Cardiol 1985;55:1645.
39. Martinez-Naharro A, Gonzalez-Lopez E, Corovic A, et al. High prevalence of intracardiac thrombi in cardiac amyloidosis. J Am Coll Cardiol 2019;73:1733-4.
40. El-Am EA, Dispenzieri A, Melduni RM, et al. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol 2019;73:589-97.
41. Loungani RS, Rehorn MR, Geurink KR, et al. Outcomes following cardioversion for patients with cardiac amyloidosis and atrial fibrillation or atrial flutter. Am Heart J 2020;222:26-9.
42. Tan NY, Mohsin Y, Hodge DO, et al. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol 2016;27:1167-73.
43. Barbhaiya CR, Kumar S, Baldinger SH, et al. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm 2016;13:383-90.
44. Gillmore JD, Damy T, Fontana M, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018;39:2799-806.
45. Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines, and the heart rhythm society. J Am Coll Cardiol 2019;74:932-87.
46. Donnellan E, Wazni OM, Saliba WI, et al. Cardiac devices in patients with transthyretin amyloidosis: impact on functional class, left ventricular function, mitral regurgitation, and mortality. J Cardiovasc Electrophysiol 2019;30:2427-32.
47. Dubrey S, Pollak A, Skinner M, Falk RH. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Br Heart J 1995;74:541-4.
48. Cappelli F, Tini G, Russo D, et al. Arterial thrombo-embolic events in cardiac amyloidosis: a look beyond atrial fibrillation. Amyloid 2021;28:12-8.
49. Yarmohammadi H, Varr BC, Puwanant S, et al. Role of CHADS2 score in evaluation of thromboembolic risk and mortality in patients with atrial fibrillation undergoing direct current cardioversion (from the ACUTE Trial Substudy). Am J Cardiol 2012;110:222-6.
50. Donnellan E, Elshazly MB, Vakamudi S, et al. No association between CHADS-VASc score and left atrial appendage thrombus in patients with transthyretin amyloidosis. JACC Clin Electrophysiol 2019;5:1473-4.
51. Feng D, Syed IS, Martinez M, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009;119:2490-7.
52. Santarone M, Corrado G, Tagliagambe LM, et al. Atrial thrombosis in cardiac amyloidosis: diagnostic contribution of transesophageal echocardiography. J Am Soc Echocardiogr 1999;12:533-6.
53. Parsons C, Patel SI, Cha S, et al. CHA2DS2-VASc score: a predictor of thromboembolic events and mortality in patients with an implantable monitoring device without atrial fibrillation. Mayo Clin Proc 2017;92:360-9.
54. Ternacle J, Krapf L, Mohty D, et al. Aortic stenosis and cardiac amyloidosis: JACC review topic of the week. J Am Coll Cardiol 2019;74:2638-51.
55. Galat A, Guellich A, Bodez D, et al. Aortic stenosis and transthyretin cardiac amyloidosis: the chicken or the egg? Eur Heart J 2016;37:3525-31.
56. Treibel TA, Fontana M, Gilbertson JA, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging 2016;9:e005066.
57. Sperry BW, Jones BM, Vranian MN, Hanna M, Jaber WA. Recognizing transthyretin cardiac amyloidosis in patients with aortic stenosis: impact on prognosis. JACC Cardiovasc Imaging 2016;9:904-6.
59. Monticelli FC, Kunz SN, Keller T, Bleiziffer S. Cardiac amyloidosis as a potential risk factor for transapical transcatheter aortic valve implantation. J Card Surg 2014;29:623-4.
60. Moreno R, Dobarro D, López de Sá E, et al. Cause of complete atrioventricular block after percutaneous aortic valve implantation: insights from a necropsy study. Circulation 2009;120:e29-30.
61. Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 1998;91:141-57.
62. Varr BC, Zarafshar S, Coakley T, et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 2014;11:158-62.
63. Goldsmith YB, Liu J, Chou J, Hoffman J, Comenzo RL, Steingart RM. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol 2009;104:990-4.
64. Palladini G, Malamani G, Cò F, et al. Holter monitoring in AL amyloidosis: prognostic implications. Pacing Clin Electrophysiol 2001;24:1228-33.
65. Giancaterino S, Urey MA, Darden D, Hsu JC. Management of arrhythmias in cardiac amyloidosis. JACC Clin Electrophysiol 2020;6:351-61.
66. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793-867.
67. Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2018;15:e190-252.
68. Ponikowski P, Voors AA, Anker SD, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200.
69. Hamon D, Algalarrondo V, Gandjbakhch E, et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol 2016;222:562-8.
70. Lin G, Dispenzieri A, Kyle R, Grogan M, Brady PA. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol 2013;24:793-8.
71. Kristen AV, Dengler TJ, Hegenbart U, et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 2008;5:235-40.
72. Sayed RH, Rogers D, Khan F, et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J 2015;36:1098-105.
73. Escher F, Senoner M, Doerler J, et al. When and how do patients with cardiac amyloidosis die? Clin Res Cardiol 2020;109:78-88.
74. Damy T, Jaccard A, Guellich A, et al. Identification of prognostic markers in transthyretin and AL cardiac amyloidosis. Amyloid 2016;23:194-202.
75. Donnellan E, Wazni OM, Hanna M, Kanj M, Saliba WI, Jaber WA. Cardiac resynchronization therapy for transthyretin cardiac amyloidosis. J Am Heart Assoc 2020;9:e017335.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Porcari A, Pagura L, Varrà GG, Rossi M, Longo F, Saro R, Barbisan D, Cittar M, Rapezzi C, Merlo M. Grey zones in the supportive treatments of cardiac amyloidosis. Vessel Plus 2022;6:33. http://dx.doi.org/10.20517/2574-1209.2021.134
AMA Style
Porcari A, Pagura L, Varrà GG, Rossi M, Longo F, Saro R, Barbisan D, Cittar M, Rapezzi C, Merlo M. Grey zones in the supportive treatments of cardiac amyloidosis. Vessel Plus. 2022; 6: 33. http://dx.doi.org/10.20517/2574-1209.2021.134
Chicago/Turabian Style
Porcari, Aldostefano, Linda Pagura, Guerino Giuseppe Varrà, Maddalena Rossi, Francesca Longo, Riccardo Saro, Davide Barbisan, Marco Cittar, Claudio Rapezzi, Marco Merlo. 2022. "Grey zones in the supportive treatments of cardiac amyloidosis" Vessel Plus. 6: 33. http://dx.doi.org/10.20517/2574-1209.2021.134
ACS Style
Porcari, A.; Pagura L.; Varrà GG.; Rossi M.; Longo F.; Saro R.; Barbisan D.; Cittar M.; Rapezzi C.; Merlo M. Grey zones in the supportive treatments of cardiac amyloidosis. Vessel Plus. 2022, 6, 33. http://dx.doi.org/10.20517/2574-1209.2021.134
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 10 clicks
Cite This Article 10 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.