Cardiac amyloidosis: a changing epidemiology with open challenges
Abstract
Cardiac amyloidosis (CA) is increasingly diagnosed due to the advancements made in diagnostics and therapeutics in the last decades, particularly in the field of transthyretin-related CA. Studies that have used bone scintigraphy for screening at-risk conditions have shown that about one out of ten patients with heart failure with preserved ejection fraction (HFpEF), aortic stenosis undergoing valve replacement, or hypertrophic cardiomyopathy (HCM) diagnosed later in life might have an underlying or concomitant CA. At the same time, the epidemiology of these conditions is also rapidly evolving. HFpEF has become the leading form of heart failure, and HFpEF patients are increasingly cared for in non-cardiology settings due to their older age and substantial burden of comorbidities. Aortic stenosis is increasingly treated percutaneously at earlier stages of the disease, determining a significant gain in survival. Hypertrophic cardiomyopathy is nowadays mostly diagnosed in middle-aged adults with near-normal life expectancy, with a greater chance of misdiagnosing CA as HCM or of an overlap between the two conditions. In all these contexts, the therapeutic and prognostic implications of diagnosing CA will have to be further investigated. Meanwhile, the diagnostic workup of patients with suspected CA should always be completed with the systematic exclusion of a plasma cell dyscrasia, the acquisition of tomographic imaging at bone scintigraphy, and the completion of genetic testing for transthyretin-related forms.
Keywords
INTRODUCTION
Cardiac amyloidosis (CA) is an infiltrative disease of the myocardium, in which different proteins may deposit within the myocardial interstitium[1] causing an increase in cardiac wall thickness, progressive cardiac dysfunction and heart failure (HF), conduction disturbances, and arrhythmias[2]. The two most prevalent forms of CA are due to the deposition of: (1) immunoglobulin light chains (AL) produced from plasma cells in the context of plasma cell dyscrasia (among which is multiple myeloma)[3]; and (2) transthyretin (TTR)[4]. Transthyretin-related CA (ATTR-CA) is a result of either a genetic mutation in the gene coding for this protein (ATTRv-CA, variant) or advanced age (ATTRwt-CA, wild-type or senile), which determines TTR tetramer dimerization and subsequent formation of aggregation of monomers within the myocardium.
In this narrative review, we summarize the most recent evidence concerning the changing epidemiology of CA. Then, some final remarks are made concerning open challenges in its diagnostic workup and therapeutic management, highlighting the importance of adhering to the recommended CA diagnostic algorithm to avoid misdiagnosis and discussing the opportunity of screening populations at risk of CA to initiate disease-specific treatments. Pertinent articles were identified through a MEDLINE search of the English language literature up to April 2021, followed by a manual selection of manuscripts discussing the abovementioned topics and considered most relevant by the authors.
WHAT IS KNOWN ABOUT THE EPIDEMIOLOGY OF CARDIAC AMYLOIDOSIS
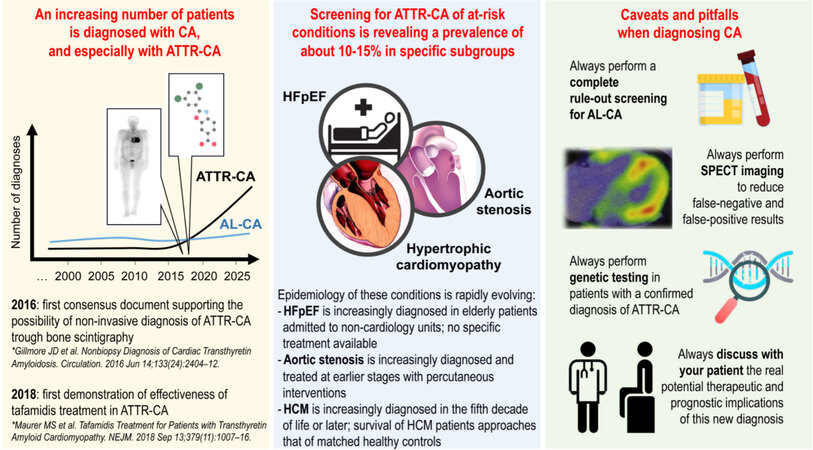
Systemic amyloidosis is listed among rare diseases within international rare diseases’ databases such as Orphanet (www.orpha.net) and NORD (https://rarediseases.org). A prevalence lower than 1/2000 has been estimated for AL and ATTR amyloidosis, which is why both conditions are nowadays considered rare. Nonetheless, when one refers specifically to amyloidosis with cardiac involvement, updated epidemiological data are lacking (as discussed below), and it is in the opinion of renowned experts worldwide that the prevalence of some forms of CA, including AL-CA and ATTRwt-CA, might actually be higher than it is usually believed[3,4]. CA is, indeed, difficult and challenging to diagnose, with about a third of all AL-CA patients being seen on average by more than four specialists before reaching final diagnosis[5]. Significant delays in diagnosis also exists for ATTR-CA[6]. However, the epidemiological scenario of CA is rapidly evolving and this condition is increasingly diagnosed[3,4], particularly because of two recent discoveries: first, the possibility of performing a non-invasive diagnosis of ATTR-CA in a vast majority of cases and, second, the demonstrated efficacy of specific treatments aiming at interrupting the progression of myocardial TTR infiltration. Indeed, the algorithm proposed by Gillmore et al.[7] in 2016 (and still considered the reference diagnostic standard for ATTR-CA by the most recent Position Paper of the European Society of Cardiology on CA[2]) introduced the possibility of a non-biopsy diagnosis of ATTR-CA by means of bone scintigraphy in the vast majority of cases. Two years later, the results of the Tafamidis in Transthyretin Amyloid Cardiomyopathy Clinical Trial (ATTR-ACT) were presented, showing the safety and efficacy of tafamidis - a selective TTR kinetic stabilizer - in reducing all-cause mortality and cardiovascular-related hospitalizations in heart failure (HF) patients diagnosed with ATTR-CA [Figure 1][8]. These advancements in the diagnostics and therapeutics of ATTR-CA are expected to have a positive impact on reducing diagnostic delays in AL-CA and potentially increasing its incidence and prevalence.
Figure 1. Current concepts in the evolving epidemiology of cardiac amyloidosis. ATTR-CA: Transthyretin-related-cardiac amyloidosis; HFpEF: heart failure with preserved ejection fraction; HCM: hypertrophic cardiomyopathy; SPECT: single photon emission computed tomography.
The description of the evolving epidemiology of CA remains scant in the literature so far[1,3,4,9]. Data on AL-CA from the Olmsted County from 1990 to 2015 show a stable incidence rate of 1.2 per 100,000 person-years[9]. A stable prevalence of AL-CA has also been recently reported in Italy, with data from a single-center 20-year study from Florence including 654 CA patients[10] and a multi-center 5-year Italian survey[11] including 642 CA patients both showing an exponential increase in the number of ATTR-CA diagnoses following 2016, the year of publication of the algorithm by Gillmore et al.[7]. Both works demonstrated that 60%-70% of CA cases diagnosed in 2019 were in fact ATTRwt-CA, with only the remaining 20%-30% being AL-CA. Data from the National Amyloidosis Center in London, which provides care to a large cohort in the United Kingdom, suggest that the diagnosis of ATTRwt-CA has increased exponentially there as well[12]. Administrative data from Medicare Beneficiaries in the United States of America also demonstrate that both the incidence and prevalence of CA have increased from 2000 to 2012 (no distinction between ATTR- and AL-CA was available in this analysis), particularly among male patients ≥ 65 years old presenting with new-onset HF[13]. As for ATTRv-CA, this has been considered a disease with endemic regions due to the variable geographical distribution of TTR mutations[4]. Nonetheless, the growing use of genetic testing that should follow the expanding use of bone scintigraphy will likely reveal the existence of additional endemic areas, including clusters of ATTRv-CA patients with mutations once considered to be rare[14], determining an enormous change in the apparently well-known geographical distribution of this disease. In conclusion, in the words of the experts, “although the exact prevalence of ATTR-CA is unknown, this is almost certainly the most common cause of CA”[4]. A “higher-than-expected” prevalence of ATTR-CA in some at-risk cardiovascular conditions has also been recently acknowledged within international rare diseases databases.
CARDIAC AMYLOIDOSIS IN AT-RISK CONDITIONS: HEART FAILURE, AORTIC STENOSIS AND HYPERTROPHIC CARDIOMYOPATHY
We have been learning by screening different conditions considered at risk of CA[4] that the prevalence of ATTR-CA in certain subgroups of cardiovascular patients might be higher than initially thought [Figure 1]. In particular, several studies have screened for CA patients with HF, with a particular focus on patients with HF with preserved ejection fraction (HFpEF). Among the several studies that screened for ATTR-CA mixed HF populations by means of bone scintigraphy[15,16], cardiac magnetic resonance[17], endomyocardial biopsy[18], or genetic testing[19], two pivotal ones with a prospective design and careful patients’ selection are worth noting. The two studies together screened 169 HFpEF patients of a mean age of about 80 years using bone scintigraphy[20,21] and found an average ATTR-CA prevalence of 15%. A larger multinational study is ongoing (NCT 04424914) aiming at enrolling 2500 HFpEF patients older than 60 years and with left ventricular hypertrophy, which will be screened for ATTR-CA by means of bone scintigraphy. This study will offer an updated and more reliable estimate of the prevalence of this disease in this at-risk condition.
A similar prevalence of ATTR-CA has been noticed in another group of patients considered at risk of CA, i.e., those with aortic stenosis (AS) undergoing interventional procedures of percutaneous valve implantation or surgical valve replacement. A summary of CA screening studies published in this population was recently presented by Rapezzi et al.[22] in an editorial. However, considering the existence of some overlap between study populations presented in different manuscript (see, e.g, Refs[23-26]), we pulled together data from three independent cohorts of 595 AS patients screened using bone scintigraphy[24,26,27] and estimated an overall average prevalence of ATTR-CA of 11%[28]. Notably, genetic TTR testing was not routinely performed in these studies, and none found an independent significant impact of this new ATTR-CA diagnosis on prognosis in these AS patients.
Another population considered at risk of CA is the one with hypertrophic cardiomyopathy (HCM) diagnosed later in life. This was primarily highlighted in the study by Maurizi et al.[29] investigating a cohort of 343 consecutive patients referred with an initial diagnosis of HCM at age ≥ 40 years. They demonstrated that the prevalence of CA in this sample linearly increased with the age of HCM diagnosis, ranging from 1% at ages 40-49 years to 26% above 80 years, with the large majority of cases being ATTR-CA[29]. Similarly, by screening 114 patients with unexplained left ventricular hypertrophy (LVH) using cardiac magnetic resonance and bone scintigraphy together, Cariou et al.[30] found a 27% prevalence of ATTR-CA.
Overall, the above evidence confirms that at least one out of ten patients with HFpEF, AS, or HCM diagnosed later in life might have an overlooked ATTR-CA, and systematic screening of patients with these conditions using bone scintigraphy has been proposed by experts[4].
EVOLVING CONCEPTS IN THE EPIDEMIOLOGY OF THESE AT-RISK CONDITIONS
HFpEF
Although a unifying definition of HF is still under discussion[31], in developed countries, the prevalence of known HF is generally reported at 1%-2% of the general adult population[32]. Most studies estimated that over half of patients diagnosed with HF have HFpEF and that this is rapidly becoming the most prevalent form of HF, especially in the elderly[32-34]. Number of comorbidities and non-cardiovascular etiology have generally been reported to be higher in patients with HFpEF than in those with HF and reduced ejection fraction (HFrEF)[35]. Accordingly, non-cardiovascular modes of death represent nowadays a more relevant competing risk in HFpEF than in HFrEF[36]. For these and other reasons, hospital admissions of elderly and frail HFpEF patients are increasingly reported in internal medicine/geriatric wards as compared to cardiology wards[37-42], and this gap is expected to further broaden over time[43]. Observational studies have shown that HF patients treated by non-cardiologists were older, more often female, with more comorbidities, and with HFpEF[37-42]. Although these findings need to be confirmed in larger multinational consecutive HF populations, they should be carefully considered in light of the high ATTR-CA prevalence in HFpEF discussed above. Awareness and knowledge of CA (and particularly of ATTR-CA) is rapidly expanding within the cardiology community, whereas it should be increasingly shared with internal medicine physicians and physicians from other specialties, who are apparently caring for the vast majority of the HFpEF population. This is of utmost importance when one also considers that no therapeutic solutions are currently available for patients with HFpEF[44], with the exception of sodium-glucose co-transporter 2 inhibitors[45]. The discovery of a specific and potentially treatable etiology of HFpEF such as in the case of ATTR-CA may have a significant impact on the management and prognosis of the affected patient. In relation to this, it has also been argued that the inclusion of patients with a missed diagnosis of CA in HFpEF studies might have significantly hindered the results of some recent HFpEF clinical trials and contributed to the failure of some HFpEF treatments[46]. Thus, a multistep screening strategy is today suggested in HFpEF patients, recommending those with red flags for CA to undergo both a light-chain assay and a bone scintigraphy to exclude for the presence of CA. These further advancements in knowledge should be spread within the whole community of physicians caring for the HFpEF population today. At present, cardiologists should always be involved in the management of these patients, particularly in the in-patient setting[42], offering rapid consultation and specific diagnostic and therapeutic directives.
Aortic valve stenosis
AS is the most common primary valve disease leading to surgery or catheter intervention in Europe and North America, with a growing prevalence due to the aging population. It causes hemodynamic overload of the left ventricle and secondary LVH. Particularly in older subjects, AS may be underdiagnosed, as doctors and patients explain their declining exercise capacity with age[47,48] and under-treated due to the increased surgical risk with aging. However, the advent of percutaneous intervention has significantly contributed to increasing the number of potential candidates to valve replacement therapy, whose efficacy vs. standard surgical procedure has also been recently demonstrated in lower-risk groups[49]. This expanding population of AS patients coming earlier to diagnosis and treatment could be the focus of a global effort aiming at excluding potential overlapping causes of LVH and particularly CA. Characteristics of AS candidates to valve replacement considered at risk of CA have been identified and include patients with low-voltage patterns and conduction abnormalities at electrocardiography and those with low-flow low-gradient phenotype and restrictive filling transmitral patterns at echocardiography[50]. Echocardiography remains the fundamental examination for the diagnosis of LVH, which represents a primary trigger of ATTR-CA suspicion. The diagnostic yield of echocardiography could be significantly improved today by increasing the awareness of secondary causes of LVH among cardiologists performing the test. Thus, the echocardiography laboratory is a key place for investments aimed at raising the suspicion of ATTR-CA and increasing the number of early-stage ATTR-CA diagnoses.
HCM
Once considered a disease of the young, with sudden death in athletes being its most ominous clinical manifestation, HCM is now recognized as a more common condition, which is increasingly diagnosed in older middle-aged adults[51,52]. The most recent data from the international SHaRe registry (Sarcomeric Human Cardiomyopathy Registry) including over 7000 HCM patients diagnosed between 1961 and 2019 at six American and five non-American HCM referral centers show that the mean age at HCM diagnosis has been steadily increasing in the years, and it is nowadays an average of 52 years[53]. The rate of diagnoses > 60 years increased from 9.2% before 2000 to 31.8% after 2010, and the prevalence of patients diagnosed at > 70 years reached 10.7% after 2010. In addition, this greater representation of older patients was associated with a more sporadic form of disease, milder phenotypes, and more frequent genotype-negative status[53]. When these findings are related to those previously described showing an increasing likelihood of ATTR-CA in patients diagnosed with HCM at ≥ 40 years of age, the potential for a HCM misdiagnosis and missed CA diagnosis in these patients should be carefully considered. Further complicating the matter, several works are available describing the possibility of ATTR-CA presenting with features usually related to HCM, such as asymmetric LVH primarily involving the interventricular septum and left ventricular outflow tract obstruction. A cardiac magnetic resonance study of 263 patients with a definite diagnosis of ATTR-CA found asymmetric septal LVH in 79% of cases[54], and cases of ATTR-CA patients with outflow tract obstruction at rest or during stress have been reported[55,56].
The increasing possibility of misdiagnosing HCM in patients with suspected ATTR-CA is not only due to the abovementioned changes in epidemiology of HCM and its overlapping features with ATTR-CA, but also to the progressive increase in survival observed in HCM patients, with longevity to 90 years or older[57]. This allows, as in the case of AS patients described above, for the presence of a dual pathology responsible for the LVH of these subjects, i.e., an innate sarcomeric mutation plus an age-related infiltrative disease.
The changing epidemiology of ATTR-CA intersects with these evolving concepts in the epidemiology of HFpEF, AS, and HCM. Greater attention to the diagnostic and therapeutic pathways of patients affected by these conditions is therefore warranted in the near future and should involve not only cardiologists but also all physicians taking care of these patients. Knowledge of the changing epidemiology of these conditions should allow bringing ATTR-CA patients earlier at diagnosis and treatment.
UNDERSTANDING THE EPIDEMIOLOGY OF CARDIAC AMYLOIDOSIS: CAVEATS AND PITFALLS
Screening the abovementioned at-risk conditions will likely be the key for earlier diagnosis and treatment of ATTR-CA in the next future. However, all screening procedures must strike a balance between the proportions of false positives and false negatives that they will tolerate. This is especially true for those screening procedures whose the risk-benefit ratio has not yet been fully elucidated, such as in the case of ATTR-CA. Screening procedure are often designed to avoid false negatives, because it is generally considered worse to miss a case than to do an unnecessary complete diagnostic workup. On the other end, the cost of unnecessary investigations as well as the possibility of needlessly exposing a patient to unpleasant or life-threatening procedures makes it important to keep the false alarm rate low as well[58]. These issues apply to ATTR-CA screening as well, for which some important remarks are worth being made [Figure 1].
Never forget to screen properly for AL-CA
The prognosis of AL-CA is significantly worse than that of ATTR-CA (usually in the range of months vs. years), thus delaying AL-CA diagnosis can significantly and adversely impact prognosis for affected patients[59]. Unfortunately, ATTR-CA misdiagnosis due to failure to properly exclude AL-CA in patients with a positive cardiac scintigraphy still represents a major issue[59-61]. Notably, a monoclonal gammopathy of undetermined significance might be present in up to 40% of patients with ATTR-CA[62]. In the presence of a monoclonal gammopathy, a tissue biopsy with amyloid typing is recommended to establish a diagnosis of amyloidosis. An endomyocardial biopsy is necessary to assess for cardiac involvement especially in those cases with a negative extracardiac biopsy but a high suspicion of CA[2,7]. However, this is an invasive procedure that is performed only by a few institutions and whose interpretation requires specific techniques and expertise[63]. Myocardial uptake in AL-CA represents one of the greatest issues with false-positive results at bone scintigraphy. This has been demonstrated in as many as 27% of patients with endomyocardial biopsy-confirmed AL-CA and grade 2 or 3 cardiac uptake on planar images[2,7]. The mechanisms for the differential uptake of bone tracers in ATTR-CA vs. AL-CA are unknown and may be related to microcalcifications within the amyloid tissue. Nonetheless, it is important to underline that a proper AL-CA workup should always precede the execution of bone scintigraphy in patients with suspected CA. This workup must screen for the presence of a monoclonal protein and should always include serum free light-chain levels and serum and urine protein electrophoresis with immunofixation. The combination of normal serum protein electrophoresis with immunofixation, normal urine protein electrophoresis with immunofixation, and a normal serum free light-chain ratio nearly always rules out systemic AL amyloidosis[3]. Unfortunately, the prescription of these tests is frequently incomplete. A recent work from a tertiary institution found that, among 550 patients screened for suspected CA, 174 (32%) did not undergo both serum immunofixation and serum free light-chain analysis tests, and only 219 (40%) did undergo complete testing[61]. It is important to remark that, regardless of whether the light chain is produced in large quantities or if the plasma cell clone itself is particularly aggressive, as long as it produces a monoclonal light chain, it is capable of causing AL amyloidosis[3]. This is why even an isolated alteration in the free light-chain ratio should warrant an extended hematological workup in patients with suspected CA. If any monoclonal protein is present, a biopsy of the affected organ, instead of bone scintigraphy, should be pursued to confirm or rule out a diagnosis of AL amyloidosis[59].
Issues with visual grading and need to systematically perform single photon emission computed tomography imaging
Grading systems for the degree of cardiac uptake on planar imaging at bone scintigraphy are both qualitative and semiquantitative. The qualitative score relates cardiac uptake as compared with rib bone uptake: grade 0, no uptake; grade 1, mild uptake, less than ribs; grade 2, moderate uptake, equal to ribs; grade 3, severe uptake, greater than ribs[64]. This visual grading scale has been validated for three bone tracers [99mTc-pyrophosphate (99mTc-PYP), 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), and 99mTc-hydroxymethylene diphosphonate (99mTc-HMDP)], and a grade 2 or 3 cardiac uptake, in the absence of a plasma cell dyscrasia, is today considered diagnostic of ATTR-CA without the need of performing a confirmatory endomyocardial biopsy[2,7]. Although visual grading appears adequate for diagnosis in properly selected patients, it has proved insufficient for the differential diagnosis with AL-CA in cases with an associated monoclonal gammopathy[7] and for risk stratification[65] in cases of confirmed ATTR-CA. Similar limitations apply to semiquantitative scores, which include the heart/contralateral thorax and the heart/whole body ratio. Although these parameters have been shown to provide some additional diagnostic and prognostic value[65-67], they are still based on planar imaging and rely on extracardiac sites as uptake reference. This can be a problem in the presence of significant abnormal extracardiac uptake (reported in up to 60% of patients with ATTR-CA[65,68]) and is one of the reasons the systematic use of single photon emission computed tomography (SPECT) is recommended[60,69]. SPECT acquisitions also have the advantage of better differentiating myocardial activity from blood pooling, which (beyond myocardial uptake in AL-CA) is the primary cause of false-positive results with planar images[2]. Two recent studies that together included about 1000 patients noted that nearly two-thirds of grade 2 scans were either entirely negative or equivocal with blood pooling when evaluated by SPECT[61,70]. The consequences of these errors might be detrimental, and cases have recently been presented of patients inappropriately prescribed with tafamidis after false-positive bone scans[71].
False-negative results may also occur[2], for example in patients with a previous myocardial infarction[72], or when myocardial infiltration is minimal, as in early stage disease, thus causing uptake to be below the current diagnostic detection threshold. The role of SPECT imaging in these contexts appears fundamental, but it has not been systematically investigated yet. Finally, we and others also noted that patients with typical cardiac involvement by echocardiography but certain pathogenic TTR mutations, including Phe64Leu and Val30Met, may have negative or mildly positive cardiac scintigraphy results[2,73]. In the case of great clinical suspicion of ATTRv-CA but negative bone scintigraphy results, the use of additional testing including endomyocardial biopsy should be judged on an individual basis.
The diagnostic accuracy of bone scintigraphy has to date been primarily demonstrated in highly selected patients evaluated at CA referral centers[60]. Its sensitivity and specificity are expected to significantly decrease when moving to larger populations of less selected individuals. This will soon become a very relevant issue, with patients having false-positive and false-negative results being exposed, respectively, to further inappropriate (and sometimes invasive) testing or prevented from receiving early disease-modifying treatments. Although the use of SPECT is recommended by the latest expert recommendations[55,64], this procedure is seldom performed and still not embedded in the diagnostic algorithms for CA. In the words of experts, “the systematic addition of SPECT to planar imaging could be considered to be the most important addition to the noninvasive testing pathway for CA in the last several years”[61]. Needless to say, this test should always be complemented with other imaging techniques such as advanced echocardiography and cardiac magnetic resonance, and the information obtained from different methodologies should be integrated to provide the greatest diagnostic accuracy[2].
Always perform genetic testing in confirmed ATTR-CA cases
Genetic counseling and testing in cases of ATTR-CA is recommended by multiple societies[74,75]. Nonetheless, screening of elderly populations is increasingly bringing to ATTR-CA diagnosis patients with associated HFpEF or AS who are in their eighth or ninth decade of life. These patients are frequently assumed to have a senile form of the disease, and use of genetic testing has been generally inconsistent in these populations. For example, genetic TTR testing was not routinely performed in patients diagnosed with ATTR-CA in two recent AS study[24,27], whereas in several other studies it was generally done only in patients with a positive bone scintigraphy. Nevertheless, as discussed above, it is known that some mutations may not lead to positive bone scintigraphy even in the presence of ATTRv-CA[2,73]. This possibility should be at least taken into account, especially in those patients who have negative bone scintigraphy but in whom a definitive diagnosis is not reached (i.e., “unspecified”) as it was reported in two recent studies[21,30]. Even in the context of referral center for CA, genetic testing for transthyretin mutations is currently performed in about 70% of patients with confirmed ATTR-CA[61]. Failure to test, even in elderly ATTR-CA patients, may result in missed opportunities to identify and counsel family members at risk and to offer certain therapies that are only approved for patients with ATTRv-CA.
Cost-effectiveness of diagnosing and treating ATTR-CA later in life
Patients screened because considered at risk (particularly HFpEF and AS ones) are frequently diagnosed with ATTR-CA in their eight decade of life[20,21,24,25,27,28]. In some cases, the diagnosis of ATTR-CA comes at a time when the patient has just undergone the treatment of a major cardiovascular conditions, such as AS with valve replacement[24,26,27] (which should significantly ameliorate their prognosis), or during one of multiple HF hospitalizations[20,21] (which represent indicators of poor outcomes). Not only the very old age and recent AS treatment or HF hospitalization, but also the great burden of comorbidities that usually occur in these patients might make any estimation of their life expectancy quite difficult, regardless of the presence of ATTR-CA. Prescription of costly medications in this setting is not straightforward; it should be not taken for granted but carefully evaluated and justified. In addition, these ATTR-CA populations do not exactly match those enrolled in clinical trials in which treatment with tafamidis has proven to be effective[8], and the potential prognostic impact of this therapy in these selected populations has yet to be demonstrated. On the contrary, preliminary analysis showed that patients treated with tafamidis at earlier stages of the ATTR-CA disease seemed to gain the greatest prognostic benefits[8].
There has been considerable debate regarding the costs of treatments for ATTR-CA, and particularly of tafamidis[76-78], recently labeled as “the most expensive cardiac medication in history”[79]. In the words of experts, “unreasonable pricing decisions have been made for this pharmaceutical, considerably above typically accepted cost-effectiveness thresholds”[76]. Cost-effectiveness analysis have shown that at a current list price of $225,000 annually, tafamidis would require an estimated 92.6% price reduction to meet commonly accepted cost-effectiveness standards of $100,000 per quality-adjusted life year[76]. This pricing issue also raises problems of access to treatment and its affordability, both from a patient’s perspective (especially in countries with mandatory health insurance coverage and direct participation to medical expenses) and health authorities’ perspective (US pharmaceutical spending, once starting tafamidis in all eligible patients, is estimated to increase up to 10%)[78].
Comorbidity burden, frailty, and life expectancy of newly diagnosed ATTR-CA patients, together with cost-effectiveness of new ATTR-CA treatments such as tafamidis, should be the objects of future specific studies, before the widespread approval of other pricy medications for ATTR-CA on the market. Physicians should carefully and openly discuss with each patient the expected prognostic benefit of starting these new treatments, taking into account the whole context in which the diagnosis of ATTR-CA has been made. In a moment in which drug industries are fostering their products in the market and media are fueling false expectations in the public, the discussion with our patients should always be straight and fair. The continuous consultation with CA referral centers may allow a better understanding of these issues and selection of candidates with greater chance of benefits from these new-targeted treatments.
CONCLUSIONS
ATTR-CA is rapidly becoming the most prevalent form of CA, and screening of conditions considered at risk of CA has revealed that the prevalence of ATTR-CA might be higher than initially thought. At least one out of ten patients with HFpEF, AS, or HCM diagnosed later in life might have an overlooked ATTR-CA, and nowadays the diagnosis is made mostly at later stages of the disease. The therapeutic and prognostic implications of this finding appear limited, and screening at earlier stages is warranted. Nonetheless, challenges exist in systematically optimizing the diagnostic accuracy of the screening process, which should always exclude a plasma cell dyscrasia first and then use bone scintigraphy with tomographic imaging followed by genetic testing. Earlier and more comprehensive screening programs will likely result in increasing the number of ATTR-CA patients who will be candidates to newly available disease-specific treatments with a favorable cost-benefit balance.
DECLARATIONS
Authors’ contributionsWrote the first draft: Canepa M, Vianello PF
Revised it thoroughly before submission: Canepa M, Vianello PF, Porcari A, Merlo M, Scarpa M
All authors contributed equally to the design of this manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestDr. Canepa received speaker and advisor fees from Akcea Therapeutics, Menarini, Novartis, Pfizer, Sanofi e Sanofi Genzyme, Vifor Pharma, and two investigator-initiated grants from Pfizer. Dr. Merlo received an investigator-initiated grant from Pfizer and speaker fees from Vifor Pharma. Dr. Scarpa received honoraria and/or research grant from Alexion, Azafaros, Chiesi, Takeda, Sanofi Genzyme, Ultragenix, Orchard. Other authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Giovanni B, Gustafson D, Adamson MB, Delgado DH. Hiding in plain sight: cardiac amyloidosis, an emerging epidemic. Can J Cardiol 2020;36:373-83.
2. Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2021;23:512-26.
3. Witteles RM, Liedtke M. AL amyloidosis for the cardiologist and oncologist: epidemiology, diagnosis, and management. JACC CardioOncol 2019;1:117-30.
4. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail 2019;7:709-16.
5. Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Light chain amyloidosis: patient experience survey from the amyloidosis research consortium. Adv Ther 2015;32:920-8.
6. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail 2018;24:131-3.
7. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404-12.
8. Maurer MS, Schwartz JH, Gundapaneni B, et al. ATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007-16.
9. Kyle RA, Larson DR, Kurtin PJ, et al. Incidence of AL amyloidosis in Olmsted County, Minnesota, 1990 through 2015. Mayo Clin Proc 2019;94:465-71.
10. Zampieri M, Nardi G, Del Monaco G, et al. Changes in the perceived epidemiology of amyloidosis: 20 year-experience from a Tertiary Referral Centre in Tuscany. Int J Cardiol 2021;335:123-7.
11. Tini G, Cappelli F, Biagini E, et al. Current patterns of beta-blocker prescription in cardiac amyloidosis: an Italian nationwide survey. ESC Heart Fail 2021;8:3369-74.
12. Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc 2012;1:e000364.
13. Gilstrap LG, Dominici F, Wang Y, et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service Medicare beneficiaries in the United States. Circ Heart Fail 2019;12:e005407.
14. Tini G, Vianello PF, Gemelli C, Grandis M, Canepa M. Amyloid cardiomyopathy in the rare transthyretin Tyr78Phe mutation. J Cardiovasc Transl Res 2019;12:514-6.
15. Dungu JN, Papadopoulou SA, Wykes K, et al. Afro-Caribbean heart failure in the United Kingdom: cause, outcomes, and ATTR V122I cardiac amyloidosis. Circ Heart Fail 2016;9:e003352.
16. López-Sainz Á, de Haro-Del Moral FJ, Dominguez F, et al. Prevalence of cardiac amyloidosis among elderly patients with systolic heart failure or conduction disorders. Amyloid 2019;26:156-63.
17. Takeda M, Amano Y, Tachi M, Tani H, Mizuno K, Kumita S. MRI differentiation of cardiomyopathy showing left ventricular hypertrophy and heart failure: differentiation between cardiac amyloidosis, hypertrophic cardiomyopathy, and hypertensive heart disease. Jpn J Radiol 2013;31:693-700.
18. Hahn VS, Yanek LR, Vaishnav J, et al. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail 2020;8:712-24.
19. Connors LH, Sam F, Skinner M, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation 2016;133:282-90.
20. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585-94.
21. Bennani Smires Y, Victor G, Ribes D, et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging 2016;32:1403-13.
22. Rapezzi C, Giannini F, Campo G. Aortic stenosis, transcatheter aortic valve replacement and transthyretin cardiac amyloidosis: are we progressively unraveling the tangle? Eur J Heart Fail 2021;23:259-63.
23. Castaño A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017;38:2879-87.
24. Rosenblum H, Masri A, Narotsky DL, et al. Unveiling outcomes in coexisting severe aortic stenosis and transthyretin cardiac amyloidosis. Eur J Heart Fail 2021;23:250-8.
25. Nitsche C, Scully PR, Patel KP, et al. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol 2021;77:128-39.
26. Nitsche C, Aschauer S, Kammerlander AA, et al. Light-chain and transthyretin cardiac amyloidosis in severe aortic stenosis: prevalence, screening possibilities, and outcome. Eur J Heart Fail 2020;22:1852-62.
27. Scully PR, Patel KP, Treibel TA, et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur Heart J 2020;41:2759-67.
28. Tini G, Sessarego E, Benenati S, et al. Yield of bone scintigraphy screening for transthyretin-related cardiac amyloidosis in different conditions: methodological issues and clinical implications. Eur J Clin Invest 2021;51:e13665.
29. Maurizi N, Rella V, Fumagalli C, et al. Prevalence of cardiac amyloidosis among adult patients referred to tertiary centres with an initial diagnosis of hypertrophic cardiomyopathy. Int J Cardiol 2020;300:191-5.
30. Cariou E, Bennani Smires Y, Victor G, et al. Toulouse Amyloidosis Research Network collaborators*. Diagnostic score for the detection of cardiac amyloidosis in patients with left ventricular hypertrophy and impact on prognosis. Amyloid 2017;24:101-9.
31. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352-80.
32. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342-56.
33. Steinberg BA, Zhao X, Heidenreich PA, et al. Get With the Guidelines Scientific Advisory Committee and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126:65-75.
35. Chamberlain AM, St Sauver JL, Gerber Y, et al. Multimorbidity in heart failure: a community perspective. Am J Med 2015;128:38-45.
36. Vaduganathan M, Patel RB, Michel A, et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 2017;69:556-69.
37. Di Lenarda A, Scherillo M, Maggioni AP, et al. Current presentation and management of heart failure in cardiology and internal medicine hospital units: a tale of two worlds--the TEMISTOCLE study. Am Heart J 2003;146:735.
38. Miró Ò, Gil VÍ, Martín-Sánchez FJ, et al. Research Group on Acute Heart Failure of the Spanish Society of Emergency Medicine (ICA-SEMES Research Group) Researchers. Short-term outcomes of heart failure patients with reduced and preserved ejection fraction after acute decompensation according to the final destination after emergency department care. Clin Res Cardiol 2018;107:698-710.
39. Jong P, Gong Y, Liu PP, Austin PC, Lee DS, Tu JV. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation 2003;108:184-91.
40. Uthamalingam S, Kandala J, Selvaraj V, et al. Outcomes of patients with acute decompensated heart failure managed by cardiologists versus noncardiologists. Am J Cardiol 2015;115:466-71.
41. Selim AM, Mazurek JA, Iqbal M, Wang D, Negassa A, Zolty R. Mortality and readmission rates in patients hospitalized for acute decompensated heart failure: a comparison between cardiology and general-medicine service outcomes in an underserved population. Clin Cardiol 2015;38:131-8.
42. Ricciardi E, La Malfa G, Guglielmi G, et al. Characteristics of current heart failure patients admitted to internal medicine vs. cardiology hospital units: the VASCO study. Intern Emerg Med 2020;15:1219-29.
43. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9.
44. Ponikowski P, Voors AA, Anker SD, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200.
45. Anker SD, Butler J, Filippatos G, et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451-61.
46. Oghina S, Bougouin W, Bézard M, et al. The impact of patients with cardiac amyloidosis in HFpEF trials. JACC Heart Fail 2021;9:169-78.
47. d’Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J 2016;37:3515-22.
49. Mack MJ, Leon MB, Thourani VH, et al. PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-705.
50. Griffin JM, Maurer MS. Cardiac amyloidosis in severe aortic stenosis: we can find it but what should we do? Eur J Heart Fail 2020;22:1863-5.
51. Cecchi F, Olivotto I, Betocchi S, et al. The Italian Registry for hypertrophic cardiomyopathy: a nationwide survey. Am Heart J 2005;150:947-54.
52. Maron MS, Hellawell JL, Lucove JC, Farzaneh-Far R, Olivotto I. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. Am J Cardiol 2016;117:1651-4.
53. Canepa M, Fumagalli C, Tini C, et al. Temporal trend in age at diagnosis of hypertrophic cardiomyopathy: an analysis of the share registry. Circulation 2019;140:A16286.
54. Martinez-Naharro A, Treibel TA, Abdel-Gadir A, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol 2017;70:466-77.
55. Philippakis AA, Falk RH. Cardiac amyloidosis mimicking hypertrophic cardiomyopathy with obstruction: treatment with disopyramide. Circulation 2012;125:1821-4.
56. Mookadam F, Haley JH, Olson LJ, Cikes M, Mookadam M. Dynamic left ventricular outflow tract obstruction in senile cardiac amyloidosis. Eur J Echocardiogr 2006;7:465-8.
57. Maron BJ, Casey SA, Haas TS, Kitner CL, Garberich RF, Lesser JR. Hypertrophic cardiomyopathy with longevity to 90 years or older. Am J Cardiol 2012;109:1341-7.
58. Robins LN, Marcus SC. The diagnostic screening procedure writer: a tool to develop individualized screening procedures. Med Care 1987;25:S106-22.
59. Alexander KM, Witteles RM. Management of cardiac amyloidosis: do’s and don’ts. Can J Cardiol 2020;36:444-6.
60. Hanna M, Ruberg FL, Maurer MS, et al. Cardiac scintigraphy with technetium-99m-labeled bone-seeking tracers for suspected amyloidosis: JACC review topic of the week. J Am Coll Cardiol 2020;75:2851-62.
61. Poterucha TJ, Elias P, Bokhari S, et al. Diagnosing transthyretin cardiac amyloidosis by technetium Tc 99m pyrophosphate: a test in evolution. JACC Cardiovasc Imaging 2021;14:1221-31.
62. Geller HI, Singh A, Mirto TM, et al. Prevalence of monoclonal gammopathy in wild-type transthyretin amyloidosis. Mayo Clin Proc 2017;92:1800-5.
63. Abildgaard N, Rojek AM, Møller HE, et al. Immunoelectron microscopy and mass spectrometry for classification of amyloid deposits. Amyloid 2020;27:59-66.
64. Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005;46:1076-84.
65. Hutt DF, Fontana M, Burniston M, et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur Heart J Cardiovasc Imaging 2017;18:1344-50.
66. Castano A, Haq M, Narotsky DL, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol 2016;1:880-9.
67. Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2011;4:659-70.
68. Cappelli F, Gallini C, Costanzo EN, et al. Lung uptake during 99mTc-hydroxymethylene diphosphonate scintigraphy in patient with TTR cardiac amyloidosis: An underestimated phenomenon. Int J Cardiol 2018;254:346-50.
69. Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol 2019;26:2065-123.
70. Masri A, Bukhari S, Ahmad S, et al. Efficient 1-hour technetium-99 m pyrophosphate imaging protocol for the diagnosis of transthyretin cardiac amyloidosis. Circ Cardiovasc Imaging 2020;13:e010249.
71. Poterucha TJ, Elias P, Ruberg FL, et al. False positive 99mTc-pyrophosphate scanning leading to inappropriate tafamidis prescriptions. JACC Cardiovasc Imaging 2021;14:2042-4.
72. Hussain M, Collier P, Jaber W. Value of SPECT imaging in patients with TTR-amyloid: ratios are not enough. J Nucl Cardiol 2021;28:747-9.
73. Musumeci MB, Cappelli F, Russo D, et al. Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis. JACC Cardiovasc Imaging 2020;13:1314-21.
74. Hershberger RE, Givertz MM, Ho CY, et al. Genetic evaluation of cardiomyopathy-a heart failure Society of America Practice Guideline. J Card Fail 2018;24:281-302.
75. Adams D, Suhr OB, Hund E, et al. European Network for TTR-FAP (ATTReuNET). First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol 2016;29 Suppl 1:S14-26.
76. Kazi DS, Bellows BK, Baron SJ, et al. Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation 2020;141:1214-24.
77. Gurwitz JH, Maurer MS. Tafamidis-a pricey therapy for a not-so-rare condition. JAMA Cardiol 2020;5:247-8.
78. Psotka MA. Tafamidis should be accessible for all patients with transthyretin amyloid cardiomyopathy. JACC Heart Fail 2021;9:124-6.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Canepa M, Vianello PF, Porcari A, Merlo M, Scarpa M. Cardiac amyloidosis: a changing epidemiology with open challenges. Vessel Plus 2022;6:30. http://dx.doi.org/10.20517/2574-1209.2021.106
AMA Style
Canepa M, Vianello PF, Porcari A, Merlo M, Scarpa M. Cardiac amyloidosis: a changing epidemiology with open challenges. Vessel Plus. 2022; 6: 30. http://dx.doi.org/10.20517/2574-1209.2021.106
Chicago/Turabian Style
Canepa, Marco, Pier Filippo Vianello, Aldostefano Porcari, Marco Merlo, Maurizio Scarpa. 2022. "Cardiac amyloidosis: a changing epidemiology with open challenges" Vessel Plus. 6: 30. http://dx.doi.org/10.20517/2574-1209.2021.106
ACS Style
Canepa, M.; Vianello PF.; Porcari A.; Merlo M.; Scarpa M. Cardiac amyloidosis: a changing epidemiology with open challenges. Vessel Plus. 2022, 6, 30. http://dx.doi.org/10.20517/2574-1209.2021.106
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 23 clicks
Cite This Article 23 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.