Diagnosis of cardiac murmurs in children
Abstract
Heart murmurs are frequently heard, and the murmurs are the usual cause for uncovering heart defects in pediatric patients. The murmurs are grouped into systolic murmurs, diastolic murmurs, and continuous murmurs. Cautious assessment of the murmur and associated abnormalities in physical examination are likely to produce correct diagnosis of the cause of the murmur. Sometimes it may be necessary to utilize noninvasive and invasive (rarely) investigations to achieve an accurate diagnosis. Nonetheless, such diagnostic studies may frequently be required for quantification, and before intervention either by transcatheter methods or by surgery.
Keywords
INTRODUCTION
One might ask why a paper on auscultation is included in a journal issue dealing with advances and insights into congenital heart disease. While such a question is justified, the author’s response is that such inclusion is warranted because of its value in its ability to discern intricate diagnostic dilemmas[1,2] prior to invoking the assistance of sophisticated investigative studies, namely, echocardiography, magnetic resonance imaging (MRI), computed tomography (CT), and cardiac catheterization with selective cineangiography. While the younger generation of cardiologists readily invokes the advantages of echocardiography, MRI, and CT, the author seeks to emphasize the value of carefully performed physical examination, including auscultation prior to afore-mentioned investigative studies, particularly in children. Such an assertion, however, is not applicable in neonates because (1) severe cyanotic congenital heart defects (CHDs) can exist without a cardiac murmur; (2) a loud murmur does not automatically suggest that the reason for the distress in the baby is related to a heart defect; and (3) when a murmur is detected, it is not necessarily characteristic of a specified CHD, as emphasized elsewhere[3,4]. Therefore, the discussion in this paper is mainly focused on murmur evaluation in children and not in neonates.
In this paper, the cardiac murmur is defined, the prevalence of the murmurs is cited, an approach to auscultation is presented, murmurs are classified, and the differential diagnosis of the cardiac murmurs is detailed.
Definition of cardiac murmurs
Cardiac murmurs are defined as abnormal sounds or vibrations that originate in the heart and/or large blood vessels and are usually auscultated in the precordium and/or great vessel sites. The sound frequency of the vibrations varies from 50 to 1000/s. The word “murmur” is used for abnormal vibrations of a longer period, whereas the shorter duration vibrations are termed snaps or clicks. A murmur auscultated at the peripheral vascular sites is named “bruit”; for example, carotid bruit for a sound appreciated over the carotid vessels and abdominal bruit for a sound perceived across the abdominal aorta region. Conversely, a bruit heard over the precordium is called a murmur. The pattern of normal blood flow in the cardiovascular structures is assumed to be laminar. A murmur is believed to be produced once turbulence is formed secondary to an abnormal flow pattern[5]. It is commonly thought that an audible murmur is heard when the Reynolds number is in excess of 2000[5]:
Reynolds number = rQD/u
D means blood density, Q indicates blood flow velocity (mean), r means conduit (tube) radius, and u indicates viscosity of the blood.
Prevalence of murmurs
Cardiac murmur is the most frequent abnormality by which heart disease is detected, especially in children (not neonates). Furthermore, in the author’s personal experience, murmur is the most frequent reason for calling for an appointment for a child to see a pediatric cardiologist for evaluation of the heart. On routine auscultation, cardiac murmurs in the neonates and premature infants are somewhat infrequent (< 2%). However, during careful auscultation, heart murmurs may be appreciated in excess of half of these babies, and the prevalence may be in the order of 80% in normal preterm babies[5]. In otherwise normal children and teenagers, the prevalence of cardiac murmur varies from 30% to 50%. However, most of these are functional, normal, or innocent murmurs and should not be of any concern[5]. Recent studies also indicated that the majority of the murmurs are functional murmurs whether the examination is performed for routine screening or as a part of pre-sports participation screening[6,7].
An approach to auscultation
It is essential that the patient is quiet, and the auscultation should take place in noiseless surroundings so as to ensure accurate interpretation of auscultatory findings. In young babies and toddlers, it is critical to listen to the chest first before the infant is disturbed with the examination of the liver, palpation of the femoral pulses, or even precordium. Of course, ear and throat examination should not be performed prior to auscultation. Young infants are better examined initially on the mother’s lap.
At the start, the 1st and 2nd heart sounds, clicks, and snaps should be assessed before addressing the murmur[5,8,9]. The auscultation should be performed over the four classic sites of auscultation such as: (1) apex or mitral area; (2) right upper sternal border (RUSB) or aortic area; (3) left upper sternal border (LUSB) or pulmonary area; and (4) left lower sternal border (LLSB) or tricuspid area. The left mid-sternal border (LMSB) and a site midway between the apex and LLSB should also be utilized for auscultation. Other areas as deemed appropriate for a given clinical scenario should also be used for auscultation. The author prefers to utilize the eponyms, RUSB, LUSB, LLSB, apex, and LMSB, instead of aortic area, pulmonary area, tricuspid area, mitral area, etc. The auscultation should be performed in both upright and supine positions. Both the bell and diaphragm of the stethoscope should be used for auscultation.
After the murmur is discovered, initially determine the timing of occurrence of the murmur, namely, systolic, diastolic, or continuous. Additional murmur timing of the diastolic murmurs to early, mid, and late is useful (see the classification of the murmurs below). Next, the point of greatest loudness of the murmur is appraised, if necessary, by moving the head of the stethoscope inch by inch. Any particular radiation characteristics of the murmur are then determined. The examples are: radiation into the axilla of an apical holosystolic murmur of mitral regurgitation (MR) and radiation from RUSB into the neck vessels of an ejection systolic murmur of aortic stenosis (AS). By definition, radiation of murmur means that the murmur is of equal intensity both at its origin and where it radiates to. The loudness of the murmur into grades I through VI (Levine[8]) should be appraised so that a comparison between different observers during the same visit or between examinations by the same observer at different visits [Table 1] can be made. While grading of the murmur is routinely performed by most cardiologists, the grade of the murmur does not indicate a given diagnosis or severity of the cardiac problem.
Grading of the murmurs
| Grade I - Not immediately heard Grade II - Soft, but immediately heard Grade III - Loud, but no thrill Grade IV - Associated with a thrill Grade V - Heard with the edge of the tilted stethoscope Grade VI - Heard with the stethoscope lifted away from the chest wall |
Descriptions such as “blowing” and others are not helpful in making a diagnosis because such descriptive qualities have been used to characterize several heart defects. However, the depiction of the murmur’s shape is of some value. Descriptions, namely, crescendo, decrescendo, flat, may be used to characterize the murmur. Assessment of the pitch of the murmur is also helpful, and murmur’s pitch may be low, medium, or high. The intensity of the murmur may vary with the respiration, and if such is present, it should be documented. As a rule, the murmurs arising from the right side of the heart, for instance, tricuspid regurgitation (TR), do alter with the respiratory cycle, whereas the murmurs from disturbances of the left heart structures will not exhibit a change with respiration. Other unique murmur characteristics, for example, multiple clicks within the patent ductus arteriosus (PDA) murmur and musical or vibratory nature of the innocent or functional murmur, are also recorded.
Recent reviews[7,10] on auscultation suggested that mastery of skills of auscultation acquired by training and experience is necessary to differentiate functional from pathologic murmurs, and I agree with this assessment.
Simulation-guided cardiac auscultation via Harvey(©) mannequin or other cardiac patient simulators and murmur online learning experience have been used to teach medical students and residents[11-14], and such training methods have demonstrated improvement of the overall performance of these trainees in identifying the murmurs. While these methods are increasingly used and may be practical in the current era, the author believes bedside acquisition of auscultatory skills under the supervision of an experienced auscultator is important, and simulations and online material such as these and others should serve as supplementary tools.
CLASSIFICATION OF THE MURMURS
The heart murmurs are classified into: (1) systolic; (2) diastolic; and (3) continuous murmurs. Typical features of each of these murmurs, causes of the murmurs, and differential diagnosis of each murmur will be reviewed one by one.
Systolic murmurs
Murmurs that are located in between the 1st and the 2nd heart sounds are characterized as systolic murmurs. Discussion of the murmurs in systole is confined to subjects who are not cyanotic. The approach used in the diagnosis of cyanotic infants and children is by examining the magnitude of pulmonary blood flow on a chest roentgenogram[3,4] and is not discussed in this script.
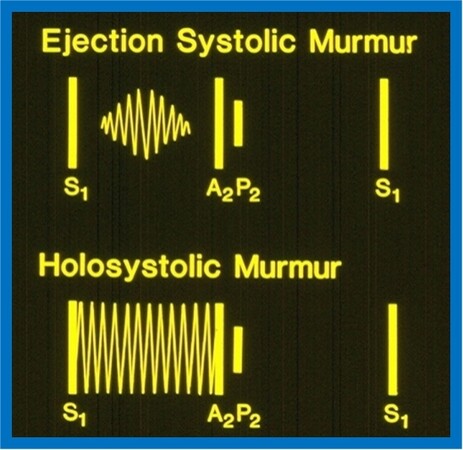
Systolic murmurs are sub-classified into: (1) Ejection systolic murmurs; and (2) holosystolic murmurs. Ejection systolic murmurs begin just following the 1st heart sound and end just prior to the onset of the 2nd sound. These murmurs are typically crescendo-decrescendo in nature and have a diamond shape (top section of Figure 1). The peaking of the murmur may occur in the early, mid, or late portions of the systole. By definition, the holosystolic murmur begins with and obscures the 1st sound and lasts through the entire systole (bottom of Figure 1). For practical purposes, the author attaches a higher value to the initial part of the definition but does not need the subsequent part to fulfill the criteria to make a diagnosis of a holosystolic murmur. Indeed, if an auscultator can distinctly appreciate the first sound separately from the murmur at the site of maximal intensity of the murmur, it may be designated as an ejection murmur. On the other hand, if the first sound and the murmur cannot be auscultated as separate entities at the site of maximal loudness of the murmur, it is designated as a holosystolic murmur. It is exceedingly important that such a distinction is undertaken since the differential diagnosis of these two murmurs is diverse and with no significant overlap, as illustrated in [Table 2] and [Table 3]. Ejection and holosystolic murmurs will be separately discussed.
Figure 1. Artist’s rendition of auscultatory findings of systolic murmurs. Ejection murmur (upper part) begins just following the first sound (S1) and stops just prior to the second sound (A2 indicates aortic component; P2 indicates pulmonary component) while a holosystolic murmur (lower part) starts with and conceals the S1 and may last through the entire systole (similar to that shown in the lower part of the diagram) or may stop prior to A2. Modified from Rao[5].
Etiology of ejection systolic murmurs. Reproduced from Rao[5]
| Common causes Aortic stenosis Coarctation of the aorta Pulmonary stenosis Atrial septal defect Functional or innocent murmur |
| Less common causes Mitral prolapse syndrome Acyanotic tetralogy of Fallot Patent ductus arteriosus |
Etiology of holosystolic murmurs. Modified from Rao[5]
| Ventricular septal defect Mitral regurgitation Tricuspid regurgitation |
Ejection systolic murmurs
The etiology of ejection systolic murmurs is shown in [Table 2]. These murmurs are classified into frequent and less frequent etiologies. The cause of the ejection systolic murmur may be ascertained by examining where the murmur is heard best and how it radiates into other sites; characteristics of the murmur, if any; character (split, not split, widely split and fixed) plus intensity of the 2nd heart sound; presence of systolic clicks; intensity of impulses in the precordium; and abnormal femoral arterial pulses [Table 4]. The findings of the electrocardiogram (ECG) and chest roentgenogram are useful in arriving at the diagnosis, and echocardiographic studies help to confirm the diagnosis.
Differential diagnosis of ejection systolic murmurs
| Point of maximal intensity of the murmur | Radiation of the murmur | Precordial impulses | Thrill | Femoral pulses | 2nd heart sound | Ejection systolic click | Chest X-ray | ECG | Echo-Doppler | Other features | |
| Aortic stenosis | RUSB LMSB | Carotid arteries | Normal or increased LV impulse | RUSB & suprasternal notch | Normal | Normal | Constant click at apex, LMSB & RUSB | Dilated ascending aorta | Normal or LVH | Thickened bicuspid aortic valve leaflets, increased Doppler flow velocity across the aortic valve | Severity of aortic stenosis is difficult to judge by clinical examination |
| Coarctation of the aorta | RUSB | Carotid arteries | Normal or increased LV impulse | Suprasternal notch | Decreased & delayed or absent | Normal | Constant click at apex, LMSB & RUSB | Inverted 3 sign on barium-filled esophagus, rib notching | Normal or left ventricular hypertrophy (LVH) | Suprasternal notch 2D echo shows coarctation, increased flow velocity in descending aorta | Measurement of blood pressure in arms and legs is helpful |
| Pulmonary stenosis | LUSB | Infra-clavicular regions & back | Normal or increased RV impulse | LSUB & suprasternal notch | Normal | Normal, diminished, or absent | LUSB LMSB LLSB, varies with respiration | Dilated main pulmonary artery | Normal or RVH | RV enlargement, increased Doppler flow velocity across the pulmonary valve | Duration & timing of peaking of the murmur, degree of splitting & intensity of 2nd sound may suggest severity of stenosis |
| Atrial septal defect | LUSB | None | Hyper-dynamic RV impulse | None | Normal | Widely split and fixed | None | Prominent main pulmonary artery, increased pulmonary blood flow | Mild RVH | Enlarged RV, paradoxical septal motion, atrial defect on subcostal echo-Doppler | Mid-diastolic murmur at LLSB |
| Functional or innocent murmur | Between apex & LLSB or at LUSB | None | Normal | None | Normal | Normal | None | Normal | Normal | Normal | Vibratory or musical quality to the murmur |
Aortic stenosis
The ejection systolic murmur of AS is appreciated best at the upper right sternal border (maybe better heard at mid left sternal border in neonates and young children). The murmur typically transmits well into both the carotid vessels. The left ventricular (LV) impulse is increased in moderate to severe AS. A thrill is palpated at the upper right sternal border and/or in the suprasternal notch. Since most AS cases are at the aortic valve level, a systolic ejection click is usually heard immediately before the murmur. This ejection systolic click is auscultated at the upper right and mid left sternal borders and apex. The click is constant and does not vary with the respiratory cycle. A normal second sound is usually heard. The arterial pulses are typically normal. The exceptions are subjects with very severe AS.
The ECG may be normal or may demonstrate LV hypertrophy. Unfortunately, neither a normal ECG or abnormal ECG findings are indicative of the severity of AS. Nevertheless, inverted T waves in leads V5 and V6 suggest severe AS. The heart size is usually normal on a chest roentgenogram, but may commonly disclose an enlarged ascending aorta, secondary to post-stenotic dilatation. Two-dimensional (2D) echocardiogram may demonstrate thickened leaflets of the aortic valve with doming. The LV fractional shortening may be increased, largely proportional to the severity of AS. The magnitude of Doppler flow velocity across the aortic valve indicates the severity of obstruction. The peak instantaneous pressure gradient across the aortic valve is calculated by using a modified Bernoulli equation:
ΔP = 4V2
Where, V is the peak Doppler velocity across the aortic valve in meters/sec, and ΔP is peak instantaneous pressure gradient in mmHg.
Patients with bicuspid aortic valves without significant AS may also have an ejection systolic click and an ejection systolic murmur. It should be noted that bicuspid aortic valve may not exhibit an audible click in infancy. At times, bicuspid aortic valve is indistinguishable from mild AS by physical examination.
Other LV outflow tract obstructions
Ejection systolic murmurs are also heard in subvalvar membranous AS, hypertrophic obstructive cardiomyopathy (HCM), and supravalvar AS. However, ejection systolic clicks are not present in these disease entities and, therefore, can be differentiated from valvar AS. In addition, the subjects with supravalvar aortic stenosis may have distinctive features of Williams syndrome such as trigonal faces, developmental delay, and infantile hypercalcemia. Furthermore, some of these patients may have a pulse or blood pressure difference between both arms. The murmur of HCM may be better auscultated at left mid sternal border and may increase in intensity with Valsalva. In patients with subaortic membrane, no clicks are present, and the murmur may be appreciated better at LLSB or LMSBs. However, 2D echo studies are useful in defining the site of LV outflow tract obstruction. The severity of obstruction can be estimated by Doppler interrogation of the LV outflow tract and supravalvar aortic region.
Coarctation of the aorta
Simultaneous palpation of femoral and brachial pulses will lead to the diagnosis of coarctation of the aorta, although the murmur is the presenting complaint. The ejection systolic murmur related to aortic coarctation may be auscultated at the right upper sternal border and is probably caused by flow disturbance across the aortic valve. The murmur of flow across aortic coarctation may be auscultated best in the left inter-scapular region over the back. The LV impulse may be prominent. A thrill is frequently felt in the suprasternal notch. Because bicuspid aortic valve is seen in a high percentage, up to 60%, of coarctation of the aorta patients, a systolic ejection click may be appreciated at the apex and upper right and mid left sternal borders. These aortic clicks do not vary with the respiratory cycle. The 2nd sound is normal in most patients with aortic coarctation. As mentioned above, the brachial and femoral artery pulses should be palpated simultaneously, and such examination demonstrates delayed and/or decreased femoral arterial pulses. The femoral artery pulses may even be absent. Measurement of blood pressures (BPs) in both the arms and one leg is recommended. A systolic blood pressure difference ≥ 20 mmHg between the arms and legs is suggestive of coarctation of the aorta. In patients who have the left subclavian artery very close to the coarctation may have diminished left brachial pulse. In subjects whose right subclavian artery has anomalous origin, below the level of aortic coarctation, will result in decreased or absent right brachial pulse. Consequently, we recommend measurement of BPs in both arms as well as palpation of both brachial pulses to discern any difference.
The ECG may demonstrate LV hypertrophy, or it may be normal. Chest X-ray abnormalities are: (1) a “3” sign in a highly penetrated chest film; (2) inverted “3” sign of the esophageal barium study; and (3) rib-notching (not in infants). 2D echocardiogram secured from the transducer placed in the suprasternal notch usually shows aortic coarctation. Doppler interrogation will demonstrate increased Doppler flow velocity; the pressure gradient across coarctation may be calculated from the flow velocity magnitude in the descending aorta (ΔP = 4V2). Association of ventricular septal defect (VSD), PDA, AS, and mitral valve abnormalities in patients with coarctation of the aorta is well known, particularly in the neonate and young infant, and therefore, the echo-Doppler studies should scrutinize for such defects.
Pulmonary stenosis
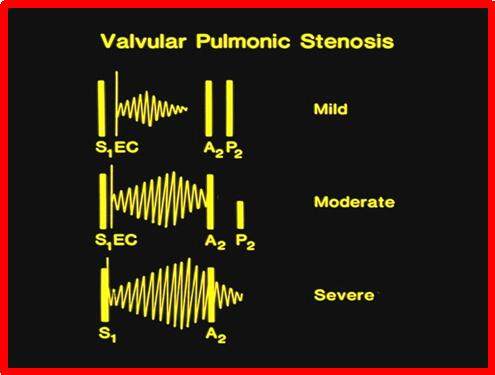
The ejection systolic murmur of pulmonary stenosis (PS) is best auscultated at upper left sternal border. The murmur transmits (radiates) into the infraclavicular areas, axillary regions, and back. The impulses of the right ventricle (RV) and RV outflow tract are prominent. A thrill is appreciated at the upper left sternal border and/or in the suprasternal notch. Since most PS patients have stenosis at the valve, an ejection click usually is heard and precedes the ejection murmur [Figure 2]. The ejection click is auscultated at lower left, mid left, and upper left sternal borders. The click changes with the respiratory cycle (decreases or becomes absent with inspiration). The location of the click can help differentiate AS from PS. The features of the 2nd sound vary with the magnitude of obstruction [Figure 2]. The BP and pulses are usually normal.
Figure 2. In valvar pulmonary stenosis, the degree of pulmonary valve obstruction may be predicted by auscultatory findings. In mild obstruction (top), the ejection click (EC) is evidently separated from the first heart sound (S1), the murmur begins with the click, peaks in early systole, and stops way before the aortic component of the second heart sound (A2), and the pulmonary component of the second heart sound (P2) is normal to increased in intensity. In moderate pulmonary stenosis (middle), the click is closer to the first heart sound, the ejection murmur peaks later in the systole and the murmur reaches the A2, and the second heart sound is widely split with a soft pulmonary component. In severe valvar stenosis (bottom), the click is either not present or occurs so close to S1 that it cannot be auscultated separately. The murmur peaks late in systole and extends beyond the A2. The second heart sound is widely split with an extremely soft or inaudible P2. Modified from Rao[5].
The relationship of click with the 1st heart sound, the degree of splitting of the 2nd sound, the loudness of the pulmonary component of the 2nd heart sound, and the length of and timing of peaking of the systolic murmur are generally indicative of the degree of PS [Figure 2]. In mild PS cases, the ejection click is evidently distinct from the 1st sound, the 2nd heart sound is normally split with normal to a slightly increased pulmonary component of the 2nd sound, and an ejection systolic murmur which peaks early in systole and terminates clearly prior to the aortic closure of the 2nd heart sound (Figure 2; top). The findings in moderate PS are an ejection systolic click that is closer to 1st heart sound than in mild PS, wide splitting of the 2nd sound with a reduced (softer) pulmonary component of the 2nd sound, and an ejection systolic murmur which peaks in mid to late systole and terminates just prior to the aortic component of the 2nd heart sound (Figure 2; middle). The characteristics of severe valvar PS (Figure 2; bottom) are an ejection systolic click which is either absent or occurs so close to the 1st sound that it is not separable from it, noticeably increased split of the 2nd heart sound with either an inaudible or a soft pulmonary component, and a prolonged ejection systolic murmur which peaks late in systole and extends way beyond the aortic closure of the 2nd sound so that the latter cannot be auscultated[15].
The loudness of the systolic murmur does not point to the severity of PS, but instead, the length of and timing of peaking of the murmur are determinants of severity of PS. The longer the murmur and the later it peaks, the more severe is the PS. Likewise, the shorter the interval between the 1st heart sound and the ejection click, the wider the split of second sound, and softer the pulmonary component, the more severe is the degree of PS[15]. Moderate and severe PS cases are associated with a precordial thrill in patients older than three to four months.
Usually, in PS, the ECG demonstrates right ventricular hypertrophy (RVH). The severity of RVH parallels the magnitude of PS. In most cases, the chest roentgenogram shows no cardiac enlargement, although a dilated main pulmonary artery (PA), post-stenotic dilatation, is frequently seen. The degree of PA dilatation has no relationship with the severity of PS. Echo-Doppler studies demonstrate enlargement of the RV, thickened and domed pulmonary valve leaflets, and increased Doppler flow velocity across the pulmonary valve. Doppler flow velocity magnitude across the pulmonary valve indicates the severity of PS. The peak instantaneous pressure gradient across the pulmonary valve may be calculated utilizing a modified Bernoulli equation:
ΔP = 4V2
Where, V is the peak velocity across the pulmonary valve in meters/sec, and ΔP is peak instantaneous pressure gradient in mmHg.
Other RV outflow tract obstructions
Ejection systolic murmurs auscultated best at LUSB are also present in patients who have infundibular PS, supravalvar PA stenosis, branch PA stenosis, and idiopathic enlargement of the main PA. Similarly, functional systolic murmurs of pulmonary ejection type are also heard best at LUSB. These entities should be differentiated from valvar PS. The characteristic ejection systolic click of valvar PS is not present in all the above, with the exception of idiopathic dilatation of the main PA. Furthermore, the murmur of infundibular PS is usually best auscultated at the lower left and mid left sternal borders. In peripheral PA stenosis, the murmur is transmitted to both axillae and back. On some occasions, the murmur is of greater intensity in the axillary region and back than at the left upper sternal border. Auscultatory findings and chest X-ray features of idiopathic dilatation of the main PA are difficult to distinguish from those of mild valvar PS. However, echo-Doppler examination is very helpful in distinguishing all the above entities.
Atrial septal defect
In patients with atrial septal defect (ASD), the systolic murmur is soft and is usually of grade II to III/VI intensity, and is heard best at LUSB. The ejection murmur is due to increased blood flow across the pulmonary valve. The RV and RV outflow tract impulses are hyper-dynamic and increased secondary to markedly increased flow across the right heart structures. Thrills are unusual in ASDs. On occasion, pulmonary ejection click is auscultated. The 2nd sound is split widely without any variation (fixed), and this finding is considered to be a distinctive feature of ASD. A mid-diastolic flow murmur of grade I to II/VI is auscultated best at the LLSB with the stethoscope’s bell. This mid-diastolic murmur is secondary to a large amount of blood flow across the tricuspid valve. Patients less than six months of age may not exhibit all the features described above. The BP and pulses are within the normal range.
The ECG demonstrates mild RVH with rSR’ pattern in the leads V4R and V1. This has been described as diastolic volume overload pattern. Mild to moderate enlargement of the heart may be seen on the chest roentgenogram. Increase in pulmonary vascular markings and dilated main pulmonary artery are also seen. Echo-Doppler studies reveal RV enlargement; some patients exhibit paradoxical inter-ventricular septal motion. The ASD can be clearly demonstrated in 2D echo, and the shunt across the ASD may be seen with pulsed and color Doppler imaging. Subcostal views are best to demonstrate the size of the ASD and the septal rims.
Other atrial level shunts
Sinus venosus and coronary sinus types of ASDs have findings similar to those of ostium secundum ASD in regard to history, physical examination, ECG, and chest roentgenogram. However, in a large proportion of patients with sinus venosus ASD, the vector of the P wave is oriented superiorly so that the P wave axis is between 0° and -90° (left axis deviation of the P wave). 2D echo demonstrates an ASD in the superior portion of the inter-atrial septum or low in the atrial septum, suggesting sinus venous ASD; this in contradistinction to secundum ASD in which the defect is situated in the mid inter-atrial septum. In coronary sinus ASDs, the defect is located close to the coronary sinus. Other defects with features similar to those of secundum ASD are ostium primum ASD, common atrium, and partial anomalous pulmonary venous connection (PAPVC). Patients with ostium primum ASD and common atrium exhibit an apical holosystolic murmur of mitral insufficiency secondary to cleft mitral valve. They also have a distinctive left axis deviation with frontal plane axis of -30° to -90° (abnormally superior ORS vector). 2D echo will demonstrate an ASD in the inferior portion of the atrial septum in the primum ASD, whereas the whole atrial septum is deficient in subjects with common atrium. The mitral cleft and mitral insufficiency can also be shown in the echo. PAPVC is difficult to differentiate from secundum ASD. PAPVC with intact atrial septum will exhibit wide but variable splitting of S2. However, careful echo-Doppler studies may identify the partial veins. Cardiac catheterization and selective cine-angiography may be needed in rare cases.
Functional or innocent murmurs
Functional, normal, or innocent murmurs are frequently heard on routine auscultation of children. Two such ejection systolic murmurs are precordial vibratory murmur and pulmonary ejection murmur.
The precordial vibratory murmur is also called Still’s murmur. This murmur is best auscultated with the stethoscope’s bell. It is typically best auscultated at a site in between the apex and lower left sternal border and does not usually transmit to other sites. Rarely, the murmur may be appreciated widely over the entire precordial area. The loudness of the murmur is between grades I and III/VI. Characteristically, this Still’s murmur has musical quality and vibratory character, and is unifrequent. When such a quality of the murmur is appreciated, one can be certain that the murmur is functional. The vibratory innocent murmur will diminish with a Valsalva maneuver.
Other findings include normal cardiac impulses, normal cardiac sounds, and normal BP and pulses. Likewise, the chest X-ray and ECG are normal. Echocardiogram is not necessary, but if performed, it is normal. Hence, the diagnosis of Still’s murmur is based on auscultatory qualities of the murmur described above as well as otherwise normal cardiovascular evaluation.
The pulmonary ejection murmur is best auscultated at the LUSB and heard better with the stethoscope’s diaphragm. The loudness of the murmur varies between grades I and III/VI. The pulmonary ejection murmur has a higher pitch than the precordial vibratory murmur. This murmur is more notably appreciated in persons who are of thin build and in patients that have “straight back syndrome”. Other findings in cardiovascular examination, namely, precordial impulses, heart sounds, BP, and brachial and femoral pulses, are within normal limits. Chest X-ray, ECG, and echocardiographic studies are also normal.
Some investigators have utilized computer-assisted auscultation[16], piezoelectric sensors[17], and artificial intelligence[18] to distinguish functional murmurs from pathologic murmurs.
Most experienced cardiologists, including the author, do not routinely perform echocardiograms in patients with the definitive clinical diagnosis of functional or innocent murmurs. However, a recent study[19] found cardiac abnormalities in three out of 62 (4.8%) patients. In addition, this study indicated that the referring physician’s expectation is that an echocardiogram is performed as part of cardiac evaluation. While the author continues to believe that echocardiogram is not necessary for patients with a clear-cut diagnosis of functional or innocent murmurs, an echo may be performed if requested by the parents or referring physician.
Other etiologies of ejection systolic murmurs [Table 2]
“Ejection” type of murmurs may also be heard in patients with mitral valve prolapse. This diamond-shaped, crescendo-decrescendo murmur is auscultated at the apex, usually late in systole. A mid-systolic click precedes the murmur. Echocardiogram is helpful in documenting mitral prolapse.
Patients with a diagnosis of acyanotic tetralogy of Fallot may have an ejection systolic murmur due to flow across the stenosed infundibulum. This murmur is usually heard at LMSB and LUSB, peaks early in systole when compared with PS with the intact ventricular septum. Pink tetralogy should be considered when the examiner hears a murmur all along the left sternal border. The RV impulse is increased, and the 2nd heart sound is single. ECG demonstrates RVH. Echo-Doppler studies demonstrate a large VSD, a large ascending aorta that overrides the inter-ventricular septum, RVH, and Doppler evidence of RV outflow tract obstruction.
Some subjects with PDA may exhibit ejection systolic murmur. These examples are either neonates or older patients with elevated PA pressures and resistance. The diastolic part of the PDA murmur is not present because of increased pulmonary vascular resistance. Multiple ejection clicks buried within the murmur, and bounding pulses are useful in the diagnosis of PDA. Echo-Doppler studies confirm the diagnosis.
Holosystolic murmurs
The etiology of holosystolic murmurs is shown in [Table 3]. Location where the murmur is heard best, where it radiates to, and change in intensity of the murmur with respiratory cycle are useful in the differential diagnosis of these defects [Table 5]. Of course, the findings of the ECG and chest roentgenogram are useful in making a diagnosis. Echo-Doppler studies are confirmatory.
Differential diagnosis of holosytolic murmurs
| Point of maximal intensity of the murmur | Radiation of the murmur | Respiratory variation of the murmur | Ventricular impulses | Other clinical findings | Chest X-ray | ECG | Echo Doppler | |
| Ventricular septal defect | LLSB | No radiation | Does not change | Increased LV and/or RV impulse | Murmur may be widely heard over the precordium. Mid-diastolic murmur at apex suggests a large shunt across the VSD | Cardiomegaly, increased pulmonary vascular markings | LVH, biventricular hypertrophy (BVH) or RVH | VSD can be imaged by 2D echo. Doppler flow velocity across the VSD is helpful in assessing the size of VSD |
| Mitral regurgitation | Apex | Radiation to anterior or mid-axillary line | Does not change | Increased LV impulse | Mid-diastolic murmur at apex suggests moderate to severe mitral insufficiency | Cardiomegaly, left atrial enlargement (LAE), normal pulmonary vascular markings | LAE, LVH | Color Doppler evidence for mitral insufficiency |
| Tricuspid regurgitation | LLSB | No radiation | Increases with inspiration | Increased RV impulse | Murmur sounds “superficial”. Prominent V-waves in jugular veins, prominent systolic hepatic pulsations | Cardiomegaly, large right atrium | RVH, right atrial enlargement (RAE) | Color Doppler evidence for tricuspid insufficiency |
Ventricular septal defect
Holosystolic murmurs of VSD are auscultated best at the lower left sternal border; these murmurs do not radiate. However, the murmur may be auscultated widely across the entire precordial area in some patients. This murmur does not exhibit respiratory variation. This murmur is produced by blood flow across the VSD during systole. In subjects with extremely small ventricular defects, the murmur may be auscultated best at the mid left and rarely at upper left[2] sternal borders. The murmur usually varies between grades II and V/VI in intensity. The loudness of murmur has no relationship with the diameter of the VSD. In patients who have small VSDs, the murmur begins with the 1st heart sound, but does not last through the whole systole. The briefer the murmur, the smaller the VSD. Small muscular VSD may have a “squirty” or “bicycle pump” quality of murmur. Increased LV impulse may be present in moderate and large VSDs. Increase in both right and left ventricular impulses may be felt in patients with large VSD. Only increase in RV impulses is seen in patients with high PA pressures and those who have developed pulmonary vascular obstructive disease (PVOD). A thrill may be appreciated at the lower left and/or mid left sternal borders. Splitting of the 2nd sound is heard, although it may be single if there is PVOD. The second (pulmonary) component of 2nd heart sound may be normal in small and moderate-sized VSDs, but is loud in subjects with large defects with elevated pulmonary artery pressure. Clicks are uncommon in VSD patients; however, they have been described in subjects with spontaneous closure of VSDs by aneurysmal formation of the membranous ventricular septum. A mid-diastolic flow rumble of grade I to II/VI intensity is auscultated slightly internal to the apex in subjects with increased left-to-right shunts with pulmonary to systemic (Qp:Qs) flow ratio ≥ 2:1 (medium and large VSDs). This murmur is auscultated best with the stethoscope’s bell. This mid-diastolic flow rumble is secondary to augmented blood flow via the mitral valve. The BP and peripheral pulses are usually normal.
The ECG shows no abnormalities in small VSDs. Evidence for mild LV hypertrophy is seen in moderate-sized VSDs. Biventricular hypertrophy has seen large VSDs. RVH is present in large defects with high pulmonary artery pressures and those who have developed PVOD. Chest roentgenogram usually shows an enlargement of the heart and increased pulmonary blood flow; the magnitude of such abnormalities is proportionate to the diameter of the VSD. M-mode echocardiogram demonstrates the increased size of the left atrium (LA) and LV; these changes are again are proportional to the size of the VSD[20]. The position of the VSD in the ventricular septum and the size of the VSD can be imaged by 2D echocardiography. Left-to-right shunting across the VSD can be shown on color Doppler imaging. Doppler flow velocity magnitudes across the VSD are useful in estimating the size of the VSD and the PA pressure.
Mitral regurgitation
The holosystolic murmur of MR is best auscultated at the apical region. The murmur transmits well into the anterior or mid-axillary sites. The loudness of the murmur does not vary with respiration. The loudness of the murmur varies between grades II and IV/VI. The LV impulse is prominent and hyper-dynamic. The degree of prominence of the LV impulse is related to the magnitude of the MR. A systolic thrill may be felt at the apical region. The 2nd sound is split in a normal fashion. The pulmonary component of the 2nd sound is usually normal, although it may be loud in patients with elevated PA pressures or increased pulmonary vascular resistance. A moderately loud 3rd sound is auscultated if MR is moderate to severe in degree. A grade I-II/VI mid-diastolic flow rumble is auscultated at the apex in patients who have moderate to severe in degrees of MR. This mid-diastolic murmur is heard better with the stethoscope’s bell. Mid-diastolic murmur is indicative of large flow across the mitral valve and does not imply additional mitral stenosis. The BP and peripheral pulses are generally within normal limits.
ECG does not show abnormalities in subjects with mild MR. LA enlargement and LV hypertrophy may be seen in patients with moderate to severe MR. Chest X-ray is normal in patients with mild MR. Enlargement of the heart due to dilated LA and LV may be seen in patients with moderate to severe MR. The degree of cardiomegaly is in proportion to the magnitude of MR. M-mode and 2D echo studies are of value in quantifying the sizes of the LA and LV. As a rule, the LA and LV sizes are proportional to the amount of MR. LV shortening fraction (SF) is usually normal. In some patients, the normal SF value is due to the LV emptying into the low resistance LA instead of the aorta, which has high peripheral vascular resistance. If the LV systolic function worsens, the SF will decrease. Color Doppler study is helpful in confirming MR.
Tricuspid regurgitation
The holosystolic murmur of TR is auscultated at the lower left sternal border. Typically, the intensity of the murmur changes with the respiratory cycle (increases during inspiration) and hence is dissimilar to the murmur of ventricular septal defect. In addition, the murmur of TR sounds more “superficial” and “scratchy”. However, the TR murmur is less frequently seen than a VSD murmur. The TR murmur is heard in association with structural tricuspid valve diseases such as Ebstein’s anomaly of the tricuspid valve and dysplastic tricuspid valve. Or, it is due to functional abnormality of the tricuspid valve, related to RV dysfunction in babies with myocardial ischemia syndrome or marked elevation of PA or RV systolic pressures. Accordingly, it appears that other, more severe heart disease coexists in the subjects with TR. Increased “v” waves in the jugular venous pulse may be seen, and systolic pulsations in the liver may be felt. The RV impulse is increased. No thrills are usually felt. The character of the 2nd sound is related to the main cardiac abnormality producing TR. Mid-diastolic flow murmur of grade I-II/VI intensity is heard best at the lower left sternal border if the degree of TR is more than moderate. Mid-diastolic murmur is due to augmented blood flow via the tricuspid orifice.
ECG demonstrates RVH and frequently reflects the primary disease process. Chest roentgenogram generally shows an enlarged cardiac silhouette, and the right atrial shadow is prominent. Echo-Doppler studies show RV volume overloading. The echo findings unravel the primary disease entity. Color Doppler imaging clearly shows the TR.
Diastolic murmurs
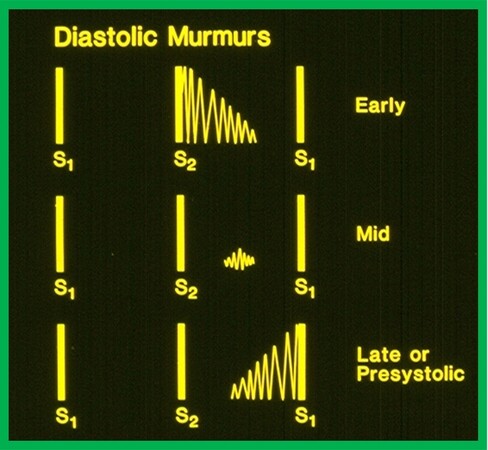
If the murmur is placed in between the 2nd and 1st heart sounds, it is described as a diastolic murmur [Figure 3]. These murmurs are classified as follows: (1) early; (2) mid; and (3) late [Figure 3]. Late diastolic murmurs are generally called presystolic murmurs. While this classification is arbitrary, such subdivision is considered clinically useful.
Figure 3. Artist’s rendition of diastolic murmurs classifying into: (1) early (top); (2) mid (middle); and (3) late or pre-systolic (bottom) diastolic murmurs are illustrated. Modified from Rao[5].
Early diastolic murmurs
Early diastolic murmurs usually have a decrescendo character (Figure 3; top) and are produced by insufficiency of aortic or pulmonary valves. Their causes are enumerated in [Table 6]. The precordial site where the murmur is heard best, murmur’s pitch, and at times, careful history and findings on physical examination are helpful in coming up with a diagnosis.
Causes of early diastolic murmurs. Modified from Rao[5]
| Aortic regurgitation Pulmonary regurgitation Pulmonary hypertension (Graham-Steel murmur) |
Aortic regurgitation
Early diastolic murmurs of aortic regurgitation (AR) have a decrescendo character and are auscultated at the RUSB and LMSBs. The murmur has a high pitch and is auscultated better with the stethoscope’s diaphragm. The murmur begins with the aortic component of the 2nd sound (Figure 3; top) and is better auscultated when the patient sits up, leans forward, and holds the breath at end-expiration. It may transmit inferiorly to the LLSB. The LV impulse is typically prominent. Diastolic thrills are rarely felt. Generally, there are no abnormal cardiac sounds. If the AR is due to a bicuspid aortic valve, an aortic systolic click is auscultated. A systolic ejection murmur is heard at upper right or at mid left sternal borders; this may be related to the increased amount of blood that has to be pumped back via the aortic valve and not due to additional AS [Figure 4]. Alternatively, the systolic component may be due to associated aortic valve stenosis.
Figure 4. Artist’s rendition of ejection systolic murmur (secondary to stenotic aortic or pulmonary valve or due to increased flow across the semilunar valve because of valvar regurgitation) and early diastolic decrescendo murmur (due to aortic or pulmonary regurgitation).
An Austin-Flint type of mid-diastolic murmur may be appreciated at the apex (please review the discussion in the section on mid-diastolic murmurs). In mild AR cases, the peripheral pulses are normally felt. But they are amplified and “bounding” in patients with moderate and severe AR. The pulse pressure is increased because of increased systolic blood pressure with concurrently decreased diastolic pressure. Peripheral signs of AR such as water-hammer pulse (quick increase and decrease of pulse when palpating the forearm), Corrigan’s pulse (strikingly augmented carotid pulses), Duroziez’s murmur [bruits (both systolic and diastolic) auscultated in the femoral artery region while it is partly blocked], pistol shot (Traube’s) sounds, and Quinke’s pulse (flushing and blanching alternatively of the capillary beds of the tips of finger) are seen in subjects with moderate to severe AR; however, these signs do not automatically identify that the AR is severe.
The ECG is normal in mild AR cases. In moderate to severe AR patients, signs of LV enlargement are seen. Chest roentgenogram reveals cardiac enlargement (mostly due to LV dilatation). Classically, there is no evidence for LA enlargement unless mitral valve disease is also present. Echo-Doppler studies demonstrate LV volume overloading. The anterior mitral leaflet may be seen to flutter on echocardiography and is indicative of AR, and corresponds to the Austin-Flint murmur described above. The LV fractional shortening is normal initially, but increases with time in response to increased volume to be pumped by the LV. With the onset of myocardial failure either due to very severe and/or prolonged AR, the LV fractional shortening decreases. Doppler study shows flow reversal in the LV outflow region. Color flow imaging unmistakably demonstrates the jet of AR, which is of use in assessing the magnitude of AR. In moderate to severe AR cases, flow reversal in the descending aorta may be observed; such findings may indicate that the AR is significant. Pressure ½ time of AR jet is useful in semi-quantification of AR.
The early diastolic murmur of AR must be distinguished from the early diastolic murmur associated with pulmonary regurgitation (PR) and pulmonary hypertension. These will be reviewed in the next section. The causes of AR are multiple, and these are: rheumatic heart disease; bicuspid aortic valve; prolapse of the aortic valve leaflet in an attempt to spontaneously close a VSD; perforation of aortic valve leaflet, which may be congenital in origin or is due to prior bacterial endocarditis; aortic root dilatation secondary to Marfan’s syndrome; aorta-to-LV tunnel, and perhaps others. It may also be due to surgical valvotomy or balloon aortic valvuloplasty for AS. Information from history, other physical findings, and echo-Doppler examination are valuable in determining the correct cause of AR.
Pulmonary regurgitation
The murmur of PR is also an early diastolic decrescendo murmur and is best auscultated at the LUSB and LMSBs. Sometimes, it radiates inferiorly to the LLSB. It is different from the murmur of AR because it is of low pitch quality in contradistinction to the high pitch of AR murmur. In addition, there are no peripheral manifestations of AR. Furthermore, the murmur of PR is best auscultated with the stethoscope’s bell, with the patient lying flat. The RV impulse is usually prominent. Heart sounds usually exhibit wide splitting of the 2nd heart sound. Single 2nd heart sound may be heard in some disease entities as reviewed in the next section. A systolic ejection murmur [Figure 4] may be appreciated at LUSB and is likely to be related to augmented volume of blood flow via the pulmonary valve, and does not automatically suggest associated PS. Nevertheless, pulmonary valve stenosis or pulmonary valve annular hypoplasia can also produce systolic murmur. The BP and pulses are unremarkable. As alluded to above, there are no peripheral signs of AR.
ECG typically demonstrates right bundle branch block, especially if the PR is due to previously operated tetralogy of Fallot. RVH may also be seen. Chest roentgenogram may either demonstrate evidence for prior surgery (for example, sternal wires) or aneurismal dilatation of pulmonary arteries (in patients with the syndrome of absent pulmonary valve). Echocardiogram will show RV volume overload in the presence of moderate to severe PR and demonstrates other heart defects. Doppler examination shows reversed Doppler flow velocity in the RV outflow tract. Color flow mapping documents the PR and helps quantify the degree of PR.
The most frequent cause of PR is prior surgical repair of tetralogy of Fallot. Surgical pulmonary valvotomy and balloon pulmonary valvuloplasty for valvar PS are the other causes. Historical information of the previous operation or catheter intervention and the scar of previous surgery are useful hints in the patient assessment. Syndrome of the absence of pulmonary valve, usually associated with other defects such as tetralogy of Fallot or VSD should also be considered in the differential. Chest X-ray may suggest aneurismal dilatation of the PAs. But echocardiogram demonstrates all the features of the syndrome, namely, VSD, pulmonary valve ring narrowing, absent or rudimentary pulmonary valve leaflets, systolic pressure gradient across the pulmonary valve, PR, and massive dilatation of the main, right, and left PAs. Cardiac catheterization and angiography are not necessary for the diagnosis, but if performed, confirm the above findings.
Pulmonary regurgitation murmur of pulmonary hypertension
The early diastolic decrescendo murmur of pulmonary hypertension, referred to as Graham Steel’s murmur, is auscultated best at LUSB and LMSBs. However, it exhibits a high pitch similar to AR murmur. Increased RV impulse, single 2nd heart sound, and ejection systolic click along the left sternal border indicative of pulmonary hypertension are also found on examination. Infrequently, a murmur of TR may be heard. While the high-pitched quality of this murmur is similar to that of AR, the location of the murmur is at the LUSB, and there are no peripheral signs of AR.
RVH is present in the ECG. Echo-Doppler studies demonstrate a dilated and hypertrophied RV. Elevated RV/PA pressures may be documented on the basis of TR and PR jet velocities. In addition, the heart defect causing pulmonary hypertension is also identified.
Mid-diastolic murmurs
Mid-diastolic murmurs (Figure 3; middle) are more difficult to appreciate because they are low-pitched murmurs, they are of low intensity (Grade I-II/VI), and are typically confined to a small part of the precordium. These murmurs are secondary to increased blood flow through a normal atrioventricular (AV) valve (during the rapid filling phage of the ventricles) or due to a normal amount of blood flow through a narrowed AV valve. The mid-diastolic murmurs related to flow across the mitral valve are auscultated better at an area somewhat internal to the apex, while those related to flow across the tricuspid valve are auscultated at the LLSB. Both murmurs are heard best when using the stethoscope’s bell for auscultation. The etiology of both AV valve mid-diastolic flow murmurs is shown in [Table 7]. There are no distinctive features of these murmurs that can differentiate the mid-diastolic murmurs from one another; however, findings in history and physical examination are useful in the differential diagnosis.
Etiology of mid-diastolic murmurs. Modified from Rao[5]
| 1. Large flow across the mitral valve (a) Ventricular septal defect (b) Patent ductus arteriosus (c) Mitral regurgitation |
| 2. Rheumatic mitral valvulitis (Carey Coombs murmur) |
| 3. Aortic regurgitation (Austin Flint murmur) |
| 4. Mitral stenosis |
| 5. Large flow across the tricuspid valve (a) Atrial septal defect (b) Anomalous pulmonary venous connection (partial or total) (c) Tricuspid regurgitation |
| 6. Tricuspid stenosis |
Large flow across the mitral valve
Increased flow across the mitral valve is produced either by augmented pulmonary blood flow secondary to heart defects such as VSD or PDA or due to moderate to severe MR (Table 7; Item 1). A holosystolic, non-radiating murmur of VSD auscultated at the lower left sternal border, a continuous murmur of PDA heard at the LUSB, or holosystolic murmur of MR auscultated at the apical region with radiation to anterior, and mid axillary lines will help differentiate them from one another.
Rheumatic mitral valvulitis
In patients with acute rheumatic fever, a mid-diastolic murmur at the apex, named Carey Coombs murmur may be auscultated. While the etiology of this murmur is not clearly established, it is generally thought that this murmur is related to the thickening of the mitral valve leaflets along with edema. Another hypothesis is that there is relative stenosis of the mitral valve produced by a dilated LV. In patients with clinically suspected rheumatic fever diagnosis, appreciation of Carey Coombs murmur is indicative of involvement of the mitral valve in the rheumatic disease process.
Austin flint murmur of aortic regurgitation
In subjects with AR, a mid-diastolic rumble at the apex, named Austin Flint murmur may be heard at the apex. This murmur is believed to result from the jet of the AR impinging on the anterior leaflet of the mitral valve, making it shudder. The presence of a high-pitched early diastolic murmur of AR at RUSB implies that this apical murmur is an Austin Flint type of murmur.
Mitral stenosis
Rheumatic and congenital mitral stenoses do result in mid-diastolic murmurs; however, typically, the mid-diastolic murmur spills into the later part of the diastole and becomes more prominent and is termed presystolic accentuation.
Increased flow across the tricuspid valve
Increased flow across the tricuspid valve is produced by (1) large shunts caused by ASD, PAPVC, or total anomalous pulmonary venous connection (TAPVC); and (2) moderate to severe TR (Table 7; Item 5). All types of ASDs (secundum, primum, sinus venosus, and coronary sinus), if they are large enough to result in Qp:Qs ratio ≥ 2:1, may have a mid-diastolic murmur auscultated best at the LLSB. Unfortunately, there are no characteristic findings of the mid-diastolic murmur that differentiates one from the other. Clinical signs of ASD, PAPVC, or TAPVC or of the murmur of TR heard best at the LLSB are useful in differentiating the etiology of mid-diastolic flow murmurs heard best at the LLSB.
Tricuspid stenosis
Congenital or rheumatic tricuspid valve narrowing, although uncommon, may result in mid-diastolic murmur heard best at lower left sternal border. There is usually a pre-systolic accentuation of the murmur.
Presystolic murmurs
Presystolic murmur (Figure 3; bottom) is infrequent in children in developed countries and is produced by stenotic AV valves. The etiology of presystolic murmurs is tabulated [Table 8]. The murmur location on auscultation and the findings in history and physical examination are useful in differentiating the causes of these murmurs.
Etiology of presystolic murmurs. Reproduced from Rao[5]
| Mitral stenosis, rheumatic and congenital Tricuspid stenosis, congenital and rheumatic Left (or right) atrial myxoma |
Mitral stenosis
Most commonly, mitral stenosis is rheumatic in origin. Mitral stenosis results in a presystolic murmur as well as a preceding low-pitched mid-diastolic murmur. The mid-diastolic murmur becomes more intense in late diastole and is generally described as presystolic accentuation. The murmur ends in S1. The murmur is produced by rapid blood flow via the stenotic mitral valve all through the atrial systole. Consequently, the murmur cannot be auscultated in subjects who are in atrial fibrillation. The murmur is better auscultated at the apical region and is best heard with the stethoscope’s bell. The murmur is better heard when the patient is in lateral decubitus. The RV impulse is usually increased, but with a normal LV impulse. Frequently, a thrill in diastole is felt at the apex. Unless carefully timed, the diastolic thrill of rheumatic mitral stenosis may be erroneously interpreted as a systolic thrill. On auscultation, the 1st heart sound is loud. The pulmonary component of the 2nd heart sound is accentuated in patients who have high PA pressures. An opening snap of high-frequency quality may be auscultated at the apex; the snap is thought to be caused by the sudden opening of the stiffened mitral valve leaflets. It is important to differentiate the opening snap from a loud 3rd sound and the pulmonary component of the 2nd sound. A systolic murmur can be heard at the apex in patients who have additional MR. The BP and arterial pulses are usually normal; however, in patients with very severe mitral stenosis, the pulse volume (pulse pressure) is decreased.
The ECG demonstrates LA enlargement and RVH. Chest roentgenogram illustrates LA dilatation, dilated main PA, and congestion of the pulmonary venous structures. Cephalization of pulmonary vascular markings and Kerley B lines, indicative of dilated lymphatics may be visualized. M-mode echo reveals decreased anterior mitral leaflet’s E to F slope and reduced movement of the posterior leaflet of the mitral valve. 2D echo helps estimation of the mitral valve area. Doppler interrogation of the LV inflow region, measuring mitral inflow gradient is useful in determining gradient through the stenotic mitral valve.
Patients who have mitral stenosis of congenital origin are likely to exhibit similar murmurs; however, the patients are clearly younger. In addition, a loud first sound is not present, and an opening snap is not heard because the mitral valve leaflets are not mobile with short and thick chordae in babies with congenital mitral stenosis. Other CHDs with comparable pathophysiology are cor triatriatum and parachute mitral valve. Such conditions do not exhibit distinctive presystolic murmur.
Tricuspid stenosis
Tricuspid valve stenoses, both congenital and rheumatic, are uncommon. Frequently, tricuspid stenosis is seen in association with other CHDs. In such situations, the clinical features are largely determined by the associated defects. Occasionally tricuspid valve may be involved in a severe rheumatic process causing tricuspid stenosis. The presystolic murmur of tricuspid stenosis is auscultated best at LLSB; the intensity of murmur increases with inspiration. In tricuspid stenosis opening snap is not commonly heard. Increased “a” waves in the jugular venous pulse and presystolic hepatic pulsations may be seen/felt. However, if there is an atrial defect decompressing the right atrium, the mentioned abnormalities are not present.
ECG shows right atrial enlargement. M-mode echo demonstrates reduced amplitude of the tricuspid valve leaflets. 2D echo may show a small tricuspid valve opening. Doppler interrogation of the RV inflow region, measuring tricuspid inflow gradient, is useful in quantifying the gradient across the tricuspid valve.
Atrial myxoma
Atrial myxomas are infrequent in the pediatric population. If present, atrial myxomas are more common in the left than in the right atrium. Embolic episodes or postural induced syncopal episodes are likely to be the presenting symptoms. Presystolic murmurs indicative of mitral valve stenosis, which varies with patient position, are suggestive of myxoma. 2D echo is an excellent method in detecting myxoma.
Continuous murmurs
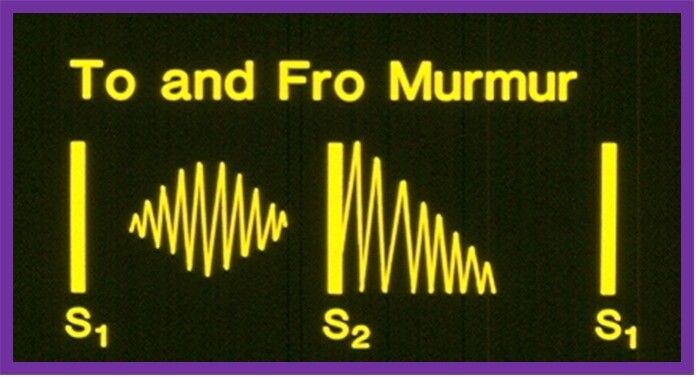
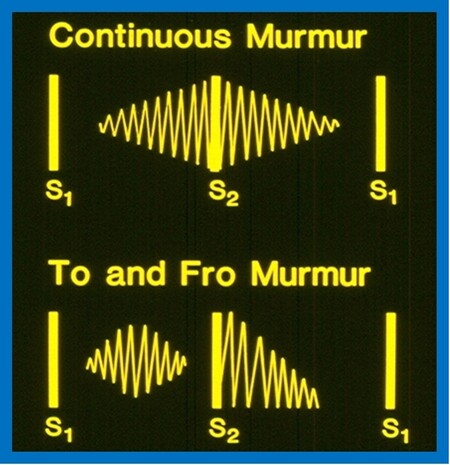
By definition, murmurs that start in systole and spill into diastole are named continuous murmurs. The systolic component of the murmur crescendos up to the 2nd heart sound whilst the diastolic part decrescendos to a variable time into the diastole (Figure 5; top).
Figure 5. Murmurs which start in systole and spill into diastole, are named continuous murmurs (top). The murmur starts during the systole, has a crescendo character until it reaches the 2nd heart sound (S2). Then, the murmur decrescendos to a variable time through the diastole. In contradistinction, a to-and-fro murmur (bottom) is composed of two separate murmurs: (1) an ejection systolic murmur; and (2) early diastolic decrescendo murmur. A distinct space between the ejection murmur and S2 is present. Modified from Rao[5].
The continuous murmurs are typically caused by blood flow from cardiac chambers or blood vessels with high pressure/resistance into venous structures with lower pressure/resistance. The blood flow occurs both during systole and diastole since there is a pressure/resistance difference during the entire cardiac cycle. The continuous murmur must be differentiated from the to-and-fro murmur (Figure 5; bottom). The latter is a mixture of systolic ejection murmur (due to aortic or pulmonary stenosis) and an early diastolic decrescendo murmur (due to aortic or pulmonary regurgitation). A distinct gap between the ejection systolic murmur and the 2nd heart sound is seen in to-and-fro murmurs (Figure 5; bottom), whereas such a gap is not seen in the continuous murmur (Figure 5; top). The etiology of continuous murmurs is shown in [Table 9]. The murmur location, changes in the murmur character with changes in body posture, and findings in history and physical examination of the primary cardiac defect are useful in the differential diagnosis.
Causes of continuous murmurs. Modified from Rao[5]
| Common causes Patent ductus arteriosus Venous hum Surgical aorto-pulmonary shunts |
| Less common causes Aorto pulmonary window Persistent truncus arteriosus Hemi truncus Embryonic collateral vessels in pulmonary atresia with ventricular septal defect Coronary arteriovenous fistula Ruptured sinus of Valsalva aneurysm Pulmonary arteriovenous fistula Peripheral pulmonary artery stenosis Coarctation of the aorta Obstructed venous return Cervical arteriovenous malformation |
Patent ductus arteriosus
The continuous murmur associated with PDA is better auscultated at the LUSB. The murmur varies from grades I to V/VI in loudness. The PDA is a frequent cause of pathologic continuous murmur. Characteristically, numerous ejection clicks within the murmur are auscultated and are considered typical for PDA. The murmur is also portrayed as a machinery murmur. Most of the time, there is no change in murmur characteristics with a change in the body position, though the diastolic part of the murmur is auscultated better when the patient is supine than when he/she is in an upright position. Nevertheless, in patients with extremely small PDAs, the continuous murmur may either disappear or become only systolic in timing in sitting-up position, but resumes to continuous type following resumption of supine posture[1]. The suggested reason for this phenomenon is “kinking” of the ductus when the patient is in the upright position[1]. The LV impulse is within normal limits in small PDAs, but it is prominent in patients who have moderate to large PDAs. A thrill is usually appreciated at the upper left sternal border and/or in the suprasternal notch. The 2nd sound is frequently within normal range; however, it is hard to be heard since it is buried in the loud continuous murmur. In patients with moderate to large PDAs, a mid-diastolic flow murmur is auscultated at the apex secondary to increased blood flow across the mitral valve. Mid-diastolic murmur of this type implies a Qp:Qs ratio ≥ 2:1. Arterial pulses are bounding in patients with moderate to large PDAs.
The ECG is normal in small PDAs. Evidence for LA and LV enlargement is seen in patients with moderate to large PDAs. Chest X-ray shows a normal-sized heart and normal pulmonary vascular markings in patients with small PDAs, while enlargement of the heart with increased pulmonary vascular markings may be present in subjects with moderate to large PDAs. LA enlargement may also be detected on the chest film. Lung collapse with secondary inflammatory changes may be seen in small babies with large PDAs. Echo-Doppler studies demonstrate near-normal-sized LA and LV in small PDAs. Enlargement of the LA and LV can be seen in patients with moderate to large PDAs; these changes are largely proportional to the size (minimal ductal diameter) of the PDA. The LV contractile function, as evaluated by LV fractional shortening and ejection fraction, is normal initially and may become hyper-contractile with time. When severe myocardial dysfunction due to prolonged and/or large shunt occurs, LV contractile function indices deteriorate. Doppler interrogation demonstrates a distinctive diastolic flow pattern in the pulmonary artery, suggestive of PDA. Color flow imaging clearly demonstrates the PDA.
Venous hum
The murmur of venous hum is also a continuous murmur; but is not due to blood flow from high pressure to low pressure cardiovascular structures (described above). It is best auscultated in the infraclavicular area, supraclavicular fossa, and at either LUSB or RUSBs. The murmur is very soft and is usually no louder than grade II/VI. The diastolic part of the murmur is a little bit louder than the systolic portion. The murmur is heard better when the patient is sitting up. The murmur fully goes away or turns out to be only systolic by compressing the veins in the neck or by rotating the patient’s head to the opposite side. The most important feature is that the murmur goes away completely in the supine position. This murmur is the most frequent of all functional heart murmurs; however, it is not frequently identified by an uninitiated auscultator. The murmur of venous hum is different from that of PDA in that it is much softer and disappears in the supine position. The other findings in cardiac evaluation are completely normal. Also, the results of the ECG, chest X-ray, if performed, are normal, as are the results of the echocardiogram.
Surgically created aorto-pulmonary shunts
A number of aorta-to-pulmonary artery shunts are created by surgery in order to augment pulmonary blood flow. All these shunt procedures exhibit continuous murmurs on auscultation. In the 1940s, anastomosis of the subclavian artery to the pulmonary artery on the same side, now called classical Blalock-Taussig (BT) operation[21] was used to increase the pulmonary blood flow. Subsequently, several other types of surgery have been used to accomplish the same purpose of augmenting the pulmonary blood flow, and these include Potts anastomosis, Waterston operation, central aorto-pulmonary shunt (directly or with a Gore-Tex graft), and modified BT shunt. In the modified BT shunt, an inter-position Gore-Tex graft is inserted between the subclavian artery and the ipsilateral PA[22]. More recently, Sano shunts[23,24], connecting the right ventricular outflow tract to the pulmonary artery with a Gore-Tex graft, have been employed to perfuse the pulmonary circulation. All the above shunts generate continuous murmurs. In practice, murmurs resulting from the surgically created shunts are difficult to differentiate from the murmur of PDA, with the exception that the multiple clicks of PDA are not auscultated in subjects who had surgically created shunts. Information regarding the surgical history and the site of the surgery scar may be useful in diagnosing the type of operation producing the continuous murmur.
Children with patent BT shunts will have continuous murmurs at the upper sternal border on the same side as the scar on the chest. On palpation, the brachial and radial pulses are reduced or not palpable in patients with classic BT shunts, while those with modified BT shunts will not have such pulse deficiency. However, it should be noted that classic BT shunts are rarely, if ever, performed at this time. Patients who had Potts and Waterston shunts will have more centrally located continuous murmurs, have either mid-sternal, left (Potts), or right (Waterston) thoracotomy scars, but it should be known that these shunts are no longer performed. The central aorto-pulmonary Gore-Tex graft shunts are typically performed via a mid-sternotomy approach, and the murmur is more medial in location, and they do not exhibit any pulse deficit. Finally, the Sano shunts usually have no or less prominent diastolic component. The majority of the patients who had been palliated with any of the above-described shunts will continue to exhibit cyanosis because the primary cyanotic CHD has not been corrected. If the shunt is large with the increased pulmonary flow, the cyanosis is mild or not clinically detectable, and such patients will have mid-diastolic flow rumble at the apex. The physical examination, chest X-ray, ECG, and echo-Doppler studies will demonstrate the primary cardiac defect.
Other causes of continuous murmur
Frequent etiologies of continuous murmur were reviewed in the preceding section. Less frequent causes of continuous murmur[5,25] are listed in (Table 9; bottom). These include: aorto-pulmonary window; truncus arteriosus; hemi truncus; multiple aorto-pulmonary collateral arteries in children with tetralogy of Fallot; rupture of sinus of Valsalva aneurysm; coronary arterio-venous fistula; pulmonary arterio-venous fistula; peripheral PA stenosis; aortic coarctation, obstructed venous return, and cervical arteriovenous malformation. Review of the clinical features and findings in chest roentgenograms and echo-Doppler studies are helpful in identifying these rare causes of continuous murmur; these were detailed elsewhere[5] for the interested reader.
SUMMARY AND CONCLUSIONS
Cardiac murmur is commonly heard on auscultation. Murmur is the frequent reason for the recognition of heart disease in children (with the exception of neonates). Mastery of skills of auscultation acquired by training and experience is important in diagnosing the causes of cardiac murmurs. Cardiac patient simulators and computer-assisted training methods have been used to educate students and residents; these methods should supplement bedside acquisition of auscultatory skills under the supervision of experienced clinicians and not become primary modes of training of our emerging physician pool. Murmurs are classified into systolic, diastolic, and continuous types. The systolic murmurs are further divided into ejection systolic and holosystolic murmurs. The more common etiologies of ejection systolic murmurs are AS, PS, ASD, coarctation of the aorta, and functional heart murmurs. The causes of holosystolic murmurs are VSD, MR, and TR. The diastolic murmurs are classified into early, mid and late (or presystolic) diastolic murmurs. The early diastolic murmurs are caused by AR, PR, and pulmonary hypertension. Mid-diastolic murmurs are produced by increased flow across the mitral valve (secondary to large shunts across a VSD or PDA or moderate to severe MR) or high flow via the tricuspid valve (secondary to ASD, partial or total anomalous pulmonary venous connection or moderate to severe TR). Other causes are Carey-Coombs murmur of rheumatic fever, Austin-Flint murmur of AR, and stenosis of the AV valves. The presystolic murmurs are produced by stenosis of the mitral or tricuspid valve and atrial myxoma. The continuous murmurs are more commonly produced by PDA, venous hum, or aorto-pulmonary shunt procedures. There are many other less common causes. Careful auscultation and other findings in history, physical examination, chest roentgenogram, and ECG will frequently help come up with an accurate diagnosis. Echo-Doppler studies are valuable and confirmatory in making the diagnosis, in quantifying the problem, and are very useful in directing the type of and timing of management.
DECLARATIONS
AcknowledgmentsThe author wishes to thank numerous patients (and their parents) whom the author had the privilege to examine at multiple institutions over the last five decades. The experience so gained resulted in formulating this review.
Authors’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Thapar MK, Rao PS, Rogers JH, Moore HV, Strong WB. Changing murmur of patent ductus arteriosus. J Pediatr 1978;92:939-41.
2. Rao PS. Auscultation is still a valid tool in the evaluation of cardiac defects in children. Congenital Cardiology Today 2018;16:1-6.
3. Rao PS. An approach to the diagnosis of cyanotic neonate for the primary care provider. Neonatology Today 2007;2:1-7.
4. Rao PS. An approach to the diagnosis of cyanotic neonate for the primary care provider. In: Rao PS, Vidyasagar D, editors. A multidisciplinary approach to perinatal cardiology. New Castle upon Tyne, UK: Cambridge Scholars Publishing; 2021. p. 294-312.
6. Austin AV, Owens DS, Prutkin JM, et al. Do 'pathologic' cardiac murmurs in adolescents identify structural heart disease? Br J Sports Med 2021:bjsports-2019.
7. Gonzalez VJ, Kyle WB, Allen HD. Cardiac examination and evaluation of murmurs. Pediatr Rev 2021;42:375-82.
8. Levine SA. Clinical heart disease. 4th ed. Philadelphia: W.B. Saunders Co.; 1951. p. 229-40.
10. Kostopoulou E, Dimitriou G, Karatza A. Cardiac murmurs in children: a challenge for the primary care physician. Curr Pediatr Rev 2019;15:131-8.
11. Butter J, McGaghie WC, Cohen ER, Kaye M, Wayne DB. Simulation-based mastery learning improves cardiac auscultation skills in medical students. J Gen Intern Med 2010;25:780-5.
12. McKinney J, Cook DA, Wood D, Hatala R. Simulation-based training for cardiac auscultation skills: systematic review and meta-analysis. J Gen Intern Med 2013;28:283-91.
13. Perlini S, Salinaro F, Santalucia P, Musca F. Simulation-guided cardiac auscultation improves medical students' clinical skills: the Pavia pilot experience. Intern Emerg Med 2014;9:165-72.
14. Power JE, Toft LEB, Barrett M. The murmur online learning experience (MOLE) curriculum improves medical students' ability to correctly identify cardiac murmurs. MedEdPORTAL 2020;16:10904.
15. Vogelpocl L, Schrire V. Auscultatory and phonocardiographic assessment of pulmonary stenosis with intact ventricular septum. Circulation 1960;22:55-72.
16. Viviers PL, Kirby JH, Viljoen JT, Derman W. The diagnostic utility of computer-assisted auscultation for the early detection of cardiac murmurs of structural origin in the periodic health evaluation. Sports Health 2017;9:341-5.
17. Takahashi K, Ono K, Arai H, Adachi H, Ito M, Kato A, Takahashi T. Detection of pathologic heart murmurs using a piezoelectric sensor. Sensors (Basel) 2021;21:1376.
19. Ip HL, Menahem S. Does echocardiography have a role in the cardiologist's diagnosis of innocent murmurs in childhood? Heart Lung Circ 2020;29:242-5.
20. Rees AH, Rao PS, Rigby JJ, Miller MD. Echocardiographic estimation of left-to-right shunt in isolated ventricular septal defects. European J Cardiol 1978;7:25-33.
21. Blalock A, Taussig HB. The surgical treatment of malformations of the heart: in which there is pulmonary stenosis or pulmonary atresia. JAMA 1945;128:189-202.
22. de Leval MR, Mckay R, Jones M, Stark J, Macartney FJ. Modified blalock-taussig shunt. J Thorac Cardovasc Surg 1981;81:112-9.
23. Sano S, Ishino K, Kawada M, et al. Right ventricle–pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2003;126:504-9.
24. Sano S, Ishino K, Kado H, et al. Outcome of right ventricle-to-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome: a multi-institutional study. Ann Thorac Surg 2004;78:1951-7; discussion 1957-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Rao PS. Diagnosis of cardiac murmurs in children. Vessel Plus 2022;6:22. http://dx.doi.org/10.20517/2574-1209.2021.105
AMA Style
Rao PS. Diagnosis of cardiac murmurs in children. Vessel Plus. 2022; 6: 22. http://dx.doi.org/10.20517/2574-1209.2021.105
Chicago/Turabian Style
Rao, P. Syamasundar. 2022. "Diagnosis of cardiac murmurs in children" Vessel Plus. 6: 22. http://dx.doi.org/10.20517/2574-1209.2021.105
ACS Style
Rao, PS. Diagnosis of cardiac murmurs in children. Vessel Plus. 2022, 6, 22. http://dx.doi.org/10.20517/2574-1209.2021.105
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 34 clicks
Cite This Article 34 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.