Coronary microvascular disease: diagnostic evaluation
Abstract
The diagnosis of coronary microvascular disease (CMD) is vital in a subset of patients with symptoms of myocardial ischemia. The typical ischemic findings on resting electrocardiogram and echocardiogram are uncommon or absent in CMD. Therefore, a high clinical suspicion is essential. We aim to review the invasive and non-invasive diagnostic testing for CMD. The goal is to assess the endothelial dysfunction related to vasoconstriction or reduced coronary blood flow reserve. Non-invasive testing includes resting electrocardiogram, exercise electrocardiogram, transthoracic color Doppler echocardiography, myocardial contrast echocardiography, positron emission tomography, and cardiac magnetic resonance imaging. Comprehensive invasive assessment includes coronary angiography followed by fractional functional flow reserve assessment, coronary flow reserve testing with intravenous acetylcholine with or without intravenous adenosine.
Keywords
INTRODUCTION
Coronary microvascular disease (CMD) is a structural and functional abnormality in the coronary microvasculature. CMD is usually secondary to vasospasm and endothelial dysfunction. However, there is accumulating evidence suggesting inflammation is playing an essential role[1-3]. CMD is more common in females[4]. A high index of clinical suspicion is necessary because CMD is associated with increased morbidity and mortality[5]. The evidence of ischemic signs of CMD on a resting electrocardiogram (ECG) and resting transthoracic echocardiography (TTE) are usually not present or may be absent. Direct visualization of coronary microvasculature is beyond the resolution of conventional imaging such as fluoroscopy, echocardiography, or computed tomography. Hence, the assessment of CMD depends on techniques to interrogating the physiology of the coronary microvasculature. Among patients with non-obstructive coronary artery disease, the diagnostic evaluation of CMD is considered while ruling out other causes, which include myocardial infarction with normal coronary arteries such as Takotsubo cardiomyopathy, spontaneous coronary artery dissection, myocarditis, and coronary artery embolism. The underlying hypothesis in diagnosing CMD is to evaluate reduced coronary vasodilatation or increased coronary vasoconstriction by measuring coronary flow reserve (CFR)/coronary flow velocity (CFV). This review discusses non-invasive and invasive approaches used to diagnose CMD (summarized in Table 1).
Coronary microvascular disease non-invasive and invasive diagnostic modalities with advantages and disadvantages
| Test | Advantages | Disadvantages |
| Resting electrocardiogram | Universal availability Cheap to perform | Do not provide information during the activity |
| Exercise electrocardiogram | Universal availability Cheap to perform Physiological test | It does not provide information on hemodynamics in the coronary vasculature |
| Hyperventilation and cold-pressor stress echocardiography | Highly specific test Easy to perform Cheap to perform | Not convenient for the patient Needs technical expertise |
| Transthoracic Doppler echocardiography | Universal availability Cheap to perform | Need technical expertise Poor body habitus precludes accurate assessment |
| Myocardial contrast echocardiography | Universal availability Cheap to perform | Needs technical expertise Cannot rule out between myocardial bridging, coronary tortuosity, and CMD |
| Multislice detector computed tomography | Quick to perform | Ionizing radiation Limitation on accurate physiologic assessment of coronary microvasculature Expensive |
| Cardiac magnetic resolution imaging | High resolution Non-invasive No radiation exposure | Expensive Needs technical expertise Risk of nephrogenic systemic fibrosis in chronic kidney disease |
| Positron emission tomography perfusion imaging | Non-invasive Most-validated Provides prognostic data | Expensive Ionizing radiation Not universally available |
| Invasive coronary angiogram pharmacologic testing | Accurate assessment of coronary physiology using pressure wires | Invasive test associated with risk of ventricular arrhythmias during pharmacologic testing Expensive |
NON-INVASIVE TESTING
Resting electrocardiogram
The resting ECG is universally available and an inexpensive test to evaluate myocardial ischemia. The resting ECG is usually normal in most patients with CMD. Therefore, ECG has poor sensitivity to establish the diagnosis of CMD. The absence of ECG changes does not exclude the diagnosis. If CMD is suspected, further workup must be pursued, as elaborated below.
Exercise electrocardiogram
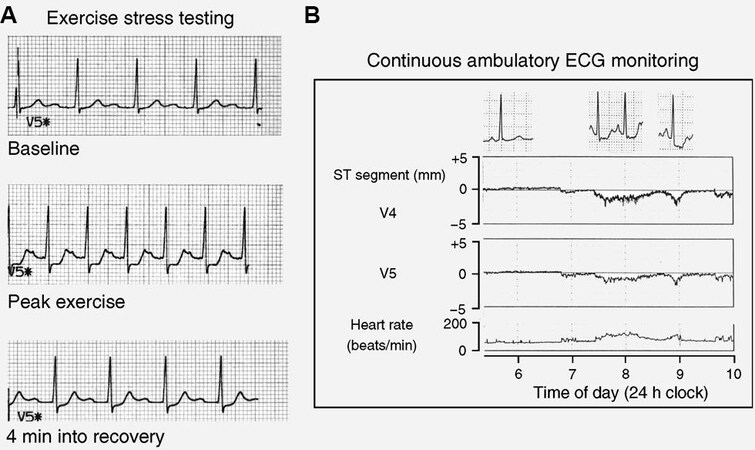
Exercise ECG is a highly specific test and is an excellent test to evaluate coronary ischemia. Exercise ECG is performed by measuring ECG at rest followed by exercise (treadmill or bike). It is cheap, widely available with good reproducibility. It also provides prognostic information based on the duration of exercise, symptoms, and ECG changes. However, it is limited by its inability to provide hemodynamics in the coronary vasculature. Therefore, an exercise ECG could be a good screening tool supplemented by other diagnostic modalities. Figure 1 shows typical ECG changes suggestive of myocardial ischemia in a patient with normal coronary arteriograms. Among 33 patients with angina of unknown cause, Chen et al.[7] demonstrated ischemia-related changes in 17 patients during a treadmill exercise test. Compared to patients who did not have symptoms on treadmill exercise test, patients with symptoms on had a significantly lower maximum great cardiac vein flow (108.8 ± 47.0 vs. 146.4 ± 23.4 mL/min, P = 0.007), higher minimum coronary vascular resistance (0.94 ± 0.41 vs. 0.61 ± 0.09 mmHg/mL/min, P = 0.003), and lower corrected great cardiac vein flow reserve (great cardiac vein flow/rate-pressure product, 0.0087 ± 0.0036 vs. 0.0125 ± 0.0019, P = 0.001) after dipyridamole infusion (0.56 mg/kg for 4 min). Chen et al.[7] concluded that CMD was likely related to effort-related angina and ischemic ECG changes during treadmill exercise testing based on these findings.
Figure 1. Electrocardiogram changes in a patient with normal coronary angiogram during exercise stress testing (A) and Holter monitor (B). Adapted from[6].
Hyperventilation and cold-pressor stress echocardiography
Hirano et al.[8] described the hyperventilation and cold-pressor stress echocardiography to detect CMD among 43 patients suspected to have vasospastic angina. It was performed by hyperventilation for 6-min
Transthoracic color Doppler echocardiography
TTE uses ultrasonic sound waves to characterize the cardiac structures. Using the Doppler principle, TTE provides a functional assessment of the valves, blood flow, and myocardium. TTE is widely available, inexpensive, and easily performed. The echocardiographic imaging of the distal left anterior descending artery was described originally by Voci et al.[10] in 25 out of 33 study participants (with coronary artery disease) and Hozumi et al.[11] in 23 participants in whom CFV was measured in the distal LAD by a Doppler guidewire at the time of coronary angiography. The middle to the distal part of LAD was imaged by color Doppler. Once imaged, a pulse Doppler was used to measure coronary blood flow, peak and mean flow velocity, deceleration time, and deceleration rate. The authors concluded that CFV reserve from transthoracic Doppler echocardiography correlated highly with those from Doppler guide wire examinations (CFV: averaged peak velocity: r = 0.97; averaged diastolic peak velocity: r = 0.97; systolic peak velocities: r = 0.97; diastolic peak velocity: r = 0.98). Galiuto et al.[12] studied this phenomenon in a small cohort of patients with cardiac syndrome X, another name for CMD, by using intravenous administration of adenosine (0.14 mg/kg/min for 90 s) followed by transthoracic echocardiography performed in the left lateral decubitus position at the cardiac apex to image the distal anteroseptal region; CFR was measured by Doppler echocardiography as an adenosine/rest velocity ratio and by myocardial contrast echocardiography as a microvascular volume, velocity, and flow adenosine/rest ratio. Compared with controls, patients with syndrome X demonstrated lower CFR, CFV, and microvascular flow reserves. Prognostically, the CFR by TTE has shown better outcomes among patients with normal CFR at 48 months follow-up[13]. A good correlation has been demonstrated between echocardiographic Doppler measurements and positron emission tomography. However, compared to positron emission tomography, echocardiographic Doppler imaging is limited by echocardiographic challenges in visualizing coronary arteries, technical expertise, and poor echo windows in many patients[14,15].
Myocardial contrast echocardiography
Myocardial contrast echocardiography (MCE) involves the intravenous administration of an ultrasound contrast agent [1.5 mL of Definity® 17 diluted in 28.5 mL of saline solution (0.09%) for a total volume of
Multislice detector computed tomography with fractional functional flow assessment
Multislice detector computed tomography involves the intravenous administration of iodinated contrast and adenosine (0.14 mg/kg/min for 5 min) to image the coronary vasculature. Images are analyzed by measuring the attenuation over time in basal and hyperemic conditions and plotting time-attenuation curves. Fractional functional flow using multislice detector computed tomography has been established to evaluate epicardial coronary occlusions. However, the current imaging does not incorporate the influence of microvasculature[17]. Therefore, multislice detector computed tomography is currently not used to evaluate CMD. Micro-optical coherence tomography, a relatively new application to visualize the endothelial cells in coronary arteries, has been described by Nishimiya et al.[18]. This may help in better understanding the physiology of coronary micro-vasculature in the future.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging (CMRI) has emerged as a good option for the non-invasive evaluation of the heart. CMRI uses strong magnetic fields to generate images of the heart and blood vessels. CMRI is also used to evaluate cardiac hemodynamics by incorporating functional assessment techniques. In patients with CMD, magnetic resonance imaging can determine coronary blood flow (CBF) during maximal hyperemia by administering vasodilators such as adenosine[19]. Panting et al.[20] described subendocardial perfusion defects after intravenous administration of adenosine in 20 patients with cardiac syndrome X. Lanza et al.[21] confirmed the utility of magnetic resonance imaging with a dobutamine stress test in 18 patients with syndrome X by showing perfusion defects in LAD artery territory. Cardiac magnetic resonance imaging has the advantages of allowing high spatial resolution, lack of ionizing radiation, functional assessment of cardiac function while allowing assessment of microvascular blood flow. Contraindications for CMRI include the risk of nephrogenic systemic fibrosis in end-stage renal disease patients, the presence of non-MRI compatible hardware in the body, and gadolinium contrast allergy. Disadvantages of CMRI also include - not being universally widely available and necessitates relevant expertise in interpreting results.
Positron emission tomography-computed tomography perfusion imaging
Positron emission tomography-computed tomography (PET-CT) is a non-invasive nuclear medicine technique involving administering a specific intravenous radio-isotopic tracer (such as 13NH3, H215O, and
Invasive testing
Coronary angiography is necessary for diagnosing CMD in terms of ruling out epicardial obstructive coronary artery disease (CAD). It is routinely performed in patients with angina and abnormal non-invasive cardiac stress test. In the absence of significant epicardial disease, a delayed distal vessel opacification of contrast may be noted. This is referred to as the coronary slow flow phenomenon[23]. For further evaluation, patients with non-obstructive CAD with stenosis < 80% and fractional functional flow reserve (FFR) > 0.80 additional testings for coronary angiography are pursued. FFR is the most validated and the gold standard to identify non-obstructive stenosis. However, in recent years, non-hyperemic intracoronary pressure assessments have gained popularity. Instantaneous wave-free pressure ratio (iFR) was evaluated in the iFR SWEDHEART, and the DEFINE-FLAIR study revealed that iFR predicted outcomes like FFR. Less invasive quantitative flow ratio and diastolic hyperemia-free ratio also demonstrated good accuracy compared to FFR[24,25]. During angiography, functional testing for CMD is performed by measuring the microvascular dysfunction with intracoronary acetylcholine and non-endothelial microvascular dysfunction with adenosine[26]. Invasive provocative testing using acetylcholine has a small risk of major complications. In a review by Ciliberti et al.[27], acetylcholine (n = 6183) administration was associated with a 1% risk of ventricular tachycardia/ventricular fibrillation and a 3% risk of transient bradycardia, 2% risk of paroxysmal or persistent atrial fibrillation. This small risk continues to limit the universal application of this test, at least in the United States.
Pharmacologic coronary endothelial and non-endothelial function testing
The pharmacologic testing for CMD involves performing a coronary angiography by administering vasodilator drugs in the coronary arteries and measuring coronary blood flow. During coronary angiography, the coronary endothelial function is assessed by injecting 50 μg acetylcholine by intracoronary route over 2 to 3 min[26]. After each injection, coronary angiography is performed. Endothelial dysfunction is diagnosed if epicardial coronary artery diameter decreases by > 20% compared with baseline. If epicardial blood vessel narrowing greater than 25% is assessed along with ischemic changes on EKG, a diagnosis of epicardial coronary artery vasoconstriction is made. If the narrowing is less than 25% but associated with ischemic EKG, a diagnosis of coronary microvasculature vasospastic angina is made. If no coronary narrowing is noted with no ischemic changes, further testing with adenosine is performed to evaluate non-endothelial causes of micro-coronary vascular disease.
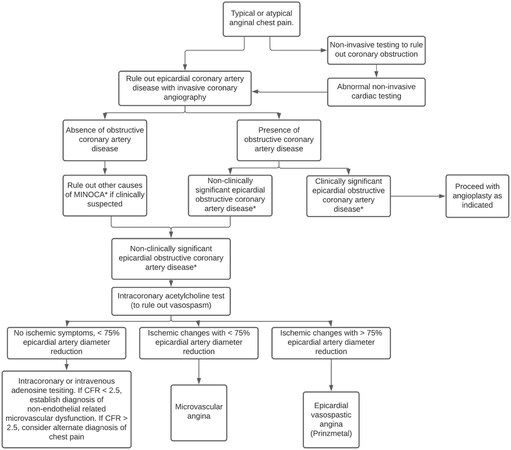
Intracoronary or intravenous adenosine is also used to measure the CFR. CFR measured during coronary angiography quantifies the CFV/CFR. Abnormal microcirculation is identified by administering adenosine during the angiogram[28]. CBF normally increases 2.5 to 5 times with adenosine compared to baseline. When there is an obstruction in the microcirculation, the CFR is reduced when measured with an intracoronary Doppler wire. Reis et al.[29] suggested a CFV reserve threshold of 2.25 as an indicator of endothelium-independent coronary microvascular dysfunction with a sensitivity of 90% and specificity of 89%. Currently, a CFR value of 2.5 is used as a convenient threshold. Patients with abnormal CFR are considered to have endothelium-independent microvascular angina[26]. According to Radico et al.[30], patients with CFR > 2.5 after adenosine should be considered to have “true” cardiac syndrome X. Figure 2 elaborates the flow chart for evaluation of CMD. In addition to doppler, intracoronary CFR measurement of thermodilution has shown to be reliable and safe. A good correlation (r = 0.79, P < 0.0001) has been demonstrated between Doppler and thermodilution derived CFR[31].
Figure 2. Flow chart for evaluation of coronary microvascular disease. It is adapted with permission from[3].
CONCLUSION
CMD is suspected when a patient has typical anginal chest pain in the absence of obstructive lesion on coronary angiography. An accurate assessment of symptoms is warranted given the higher mortality and morbidity associated with CMD. The diagnosis of CMD can be aided solely by non-invasive testing. Cardiac MRI and PET enable us to identify coronary microvascular anatomy/physiology with high spatial resolution while avoiding the need for invasive studies. While the non-invasive modalities are yet to be standardized, future studies aimed at improving the diagnostic accuracy of non-invasive testing for CMD will enable accurate, prompt, and safe diagnosis.
DECLARATIONS
Authors’ contributionsMade substantial contributions to the conception and design of the study and performed data analysis and interpretation: Voruganti D, Mehta JL
Performed data acquisition and provided administrative, technical, and material support: Voruganti D, Mehta JL
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Godo S, Takahashi J, Yasuda S, Shimokawa H. Role of inflammation in coronary epicardial and microvascular dysfunction. Eur Cardiol 2021;16:e13.
2. Sinha A, Rahman H, Perera D. Coronary microvascular disease: current concepts of pathophysiology, diagnosis and management. Cardiovasc Endocrinol Metab 2020;10:22-30.
3. Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J 2009;30:1837-43.
4. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445-53.
5. Pepine CJ, Ferdinand KC, Shaw LJ, et al. ACC CVD in Women Committee. Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol 2015;66:1918-33.
6. Kaski J, Iqbal K. Cardiac syndrome X: pathogenesis and management. Heart Metab 2008;40:30-5.
7. Chen JW, Ting CT, Chen CI, et al. Coronary microvascular dysfunction is associated with ischemic-like electrocardiogram during exercise in patients with anginal chest pain and normal coronary angiograms. Jpn Heart J 1996;37:865-78.
8. Hirano Y, Ozasa Y, Yamamoto T, et al. Hyperventilation and cold-pressor stress echocardiography for noninvasive diagnosis of coronary artery spasm. J Am Soc Echocardiogr 2001;14:626-33.
9. Shimizu H, Lee JD, Yamamoto M, Satake K, et al. [Induction of coronary artery spasm by combined cold pressor and hyperventilation test in patients with variant angina]. J Cardiol 1994;24:257-61. (in Japanese).
10. Voci P, Testa G, Plaustro G. Imaging of the distal left anterior descending coronary artery by transthoracic color-Doppler echocardiography. Am J Cardiol 1998;81:74G-8G.
11. Hozumi T, Yoshida K, Akasaka T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography. J Am Coll Cardiol 1998;32:1251-9.
12. Galiuto L, Sestito A, Barchetta S, et al. Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol 2007;99:1378-83.
13. Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol 2009;103:626-31.
14. Saraste M, Koskenvuo J, Knuuti J, et al. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol 2001;21:114-22.
15. Lethen H, P Tries H, Kersting S, Lambertz H. Validation of noninvasive assessment of coronary flow velocity reserve in the right coronary artery. A comparison of transthoracic echocardiographic results with intracoronary Doppler flow wire measurements. Eur Heart J 2003;24:1567-75.
16. Rinkevich D, Belcik T, Gupta NC, Cannard E, Alkayed NJ, Kaul S. Coronary autoregulation is abnormal in syndrome X: insights using myocardial contrast echocardiography. J Am Soc Echocardiogr 2013;26:290-6.
18. Nishimiya K, Yin B, Piao Z, et al. Micro-optical coherence tomography for endothelial cell visualization in the coronary arteries. JACC Cardiovasc Imaging 2019;12:1878-80.
19. Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging 2015;8:e002481.
20. Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002;346:1948-53.
21. Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol 2008;51:466-72.
22. Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518-27.
23. Wang X, Nie SP. The coronary slow flow phenomenon: characteristics, mechanisms and implications. Cardiovasc Diagn Ther 2011;1:37-43.
24. Moscarella E, Gragnano F, Cesaro A, et al. Coronary physiology assessment for the diagnosis and treatment of coronary artery disease. Cardiol Clin 2020;38:575-88.
25. Cesaro A, Gragnano F, Di Girolamo D, et al. Functional assessment of coronary stenosis: an overview of available techniques. Is quantitative flow ratio a step to the future? Expert Rev Cardiovasc Ther 2018;16:951-62.
26. Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015;131:1054-60.
27. Ciliberti G, Seshasai SRK, Ambrosio G, Kaski JC. Safety of intracoronary provocative testing for the diagnosis of coronary artery spasm. Int J Cardiol 2017;244:77-83.
28. Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825-32.
29. Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. J Am Coll Cardiol 1999;33:1469-75.
30. Radico F, Cicchitti V, Zimarino M, De Caterina R. Angina pectoris and myocardial ischemia in the absence of obstructive coronary artery disease: practical considerations for diagnostic tests. JACC Cardiovasc Interv 2014;7:453-63.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Voruganti D, Mehta JL. Coronary microvascular disease: diagnostic evaluation. Vessel Plus 2021;5:55. http://dx.doi.org/10.20517/2574-1209.2021.60
AMA Style
Voruganti D, Mehta JL. Coronary microvascular disease: diagnostic evaluation. Vessel Plus. 2021; 5: 55. http://dx.doi.org/10.20517/2574-1209.2021.60
Chicago/Turabian Style
Voruganti, Dinesh, Jawahar L. Mehta. 2021. "Coronary microvascular disease: diagnostic evaluation" Vessel Plus. 5: 55. http://dx.doi.org/10.20517/2574-1209.2021.60
ACS Style
Voruganti, D.; Mehta JL. Coronary microvascular disease: diagnostic evaluation. Vessel Plus. 2021, 5, 55. http://dx.doi.org/10.20517/2574-1209.2021.60
About This Article
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.