Clinico-pathophysiological considerations in coronary microvascular disorders
Abstract
Around half of the patients undergoing an elective coronary angiogram to investigate typical stable angina symptoms are found to have non-obstructive coronary arteries (defined as < 50% stenosis). These patients are younger with a female predilection. While underlying mechanisms responsible for these presentations are heterogeneous, structural and functional abnormalities of the coronary microvasculature are highly prevalent. Thus, coronary microvascular dysfunction (CMD) is increasingly recognised as an important consideration in patients with non-obstructive coronary arteries. This review will focus on primary coronary microvascular disorders and summarise the four common clinical presentation pictures which can be considered as endotypes - Microvascular Ischaemia (formerly “Syndrome X”), Microvascular Angina, Microvascular Spasm, and Coronary Slow Flow. Furthermore, the pathophysiological mechanisms associated with CMD are also heterogenous. CMD may arise from an increased microvascular resistance, impaired microvascular dilation, and/or inducible microvascular spasm, ultimately causing myocardial ischaemia and angina. Alternatively, chest pain may arise from hypersensitivity of myocardial pain receptors rather than myocardial ischaemia. These two major abnormalities should be considered when assessing an individual clinical picture, and ultimately, the question arises whether to target the heart or the pain perception to treat the anginal symptoms.
Keywords
INTRODUCTION
Around half of the patients undergoing an elective coronary angiogram to investigate suspected coronary artery disease are found to have non-obstructive coronary arteries (defined as < 50% stenosis)[1]. These patients are a clinical conundrum since many have features consistent with a history of typical angina and clinical evidence of myocardial ischaemia despite the absence of obstructive coronary artery disease to account for their symptoms. Unfortunately, many clinicians often dismiss the symptoms as “non-cardiac” in nature without further investigating other potential underlying coronary mechanisms. These include epicardial coronary artery spasm and coronary microvascular dysfunction. This review will focus on disorders associated with coronary microvascular dysfunction (CMD), particularly those considered as Primary Coronary Microvascular Disorders (also referred to as Type 1 CMD)[2] where there is no clinically overt secondary cause for the CMD (e.g., distal coronary embolisation during coronary stenting).
THE CORONARY MICROVASCULAR DISORDERS
Since the advent of coronary angiography, clinicians have been puzzled when encountered with the “Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease”[3]. The possibility of CMD being responsible for the symptoms was often empirically entertained but only attracted scientific investigation in 1973 when Arbogast & Bourassa undertook rapid atrial pacing in 11 symptomatic patients with obstructive coronary artery disease (Group C) and 10 patients with chest pain and normal angiography (Group X - experimental group); documenting that both groups experienced chest pain, ischaemic ST changes, and lactate production although the left ventricular haemodynamic responses differed[4]. This prompted the accompanying editorial to refer to the Group X patients as having “Syndrome X”[5]. Since this iconic landmark study, multiple clinical pathophysiologic studies have been undertaken to understand the underlying mechanisms responsible for these puzzling patients with unmistakable angina in the absence of obstructive coronary artery disease. Historically, these can be considered as four endotypes [Table 1], although the delineation between these endotypes is unclear and requires further investigation.
Coronary microvascular disorders pathophysiological endotypes
| Microvascular ischaemia (formerly Syndrome X) |
| · Pathophysiological concept: abnormal ischaemic markers in the absence of obstructive epicardial coronary artery disease or coronary spasm, inferring microvascular aberrations · Original criteria (Syndrome X)[32]: (1) exertional chest pain; (2) positive ETT; (3) normal coronary angiogram; (4) no evidence of coronary spasm · Avoid term “Syndrome X” since misused to describe any patient with chest pain & normal angiogram, thus utilise term “microvascular ischaemia” for patients with exertional angina, documented evidence of ischaemia, normal coronary angiogram, and no evidence of spasm |
| Impaired microvascular vasodilator response |
| · Pathophysiological concept: impaired coronary blood flow response to conventional hyperaemic stimuli (e.g., dipyridamole, adenosine, rapid pacing, and maximal exercise) · Original criteria (Microvascular angina[33]: (1) impaired coronary flow reserve < 2.0; and (2) non-obstructive coronary arteries · COVADIS Microvascular Angina definition expanded to include both markers of ischaemia and impaired coronary microvascular function (impaired CFR, microvascular spasm, or slow flow) |
| Microvascular spasm |
| · Pathophysiological concept: Ach-induced ischaemia in the absence of obstructive epicardial coronary artery disease or coronary spasm, inferring inducible microvascular spasm · Original criteria (Microvascular Spasm)[9]: (1) angina and/or ischaemic ECG changes with ACh administration without epicardial artery spasm; and (2) non-obstructive coronary arteries |
| Coronary slow flow phenomenon |
| · Pathophysiological concept: an angiographic phenomenon characterised by delayed resting contrast flow in the absence of obstructive epicardial coronary artery disease or coronary spasm, inferring increased resting coronary microvascular resistance · Original criteria (Coronary Slow Flow Phenomenon)[34]: (1) delayed opacification of the distal epicardial coronary arteries (i.e., TIMI-2 flow); and (2) non-obstructive coronary arteries · Subsequent definitions utilised TIMI-frame count thresholds[34] |
Microvascular ischaemia (formerly Syndrome X)
In the initial iteration of the term “Syndrome X”, specific clinical criteria were recommended, including exertional chest pain, ischaemic ST segment depression during exercise stress testing, normal coronary epicardial arteries on selective angiography, and the absence of coronary artery spasm [Table 1]. However, as the investigation into this disorder evolved, patients with other clinical markers ischaemia were also included (i.e., stress-induced reversible perfusion defects or transient regional wall motion abnormalities on imaging studies). Hence, the term Syndrome X incorporated patients with chest pain and evidence of ischaemia despite the absence of obstructive coronary artery disease or epicardial artery spasm, inferring that microvascular aberrations were responsible for the ischaemia (i.e., Microvascular Ischaemia). Substantial clinical studies were conducted utilising this “Syndrome X” definition, and it still represents the largest body of literature in this field.
Unfortunately, with time the term “Syndrome X” was utilised in a more generic context to describe any patient with chest pain suspicious of angina and non-obstructive coronary arteries. Furthermore, the term “Syndrome X” was also used to describe patients with metabolic syndrome, adding more confusion. Consequently, the term “Syndrome X” is now avoided in contemporary published literature, considering the ambiguity in which diagnosis is being considered and the connotations for patients as to the nature of their symptoms. Thus, for the purposes of this review, the term “microvascular ischaemia” will be used to refer to the studies that utilised the specific original criteria for “Syndrome X” [Table 1].
Microvascular angina
This term was first used to describe patients who had chest pain with an impaired coronary flow reserve (CFR, i.e., less than doubling of the coronary blood flow response to a standard hyperaemic stimulus) in the absence of obstructive coronary artery disease[6]. However, ss none of these patients had a positive exercise ECG[7], it was difficult to reconcile these findings with studies of microvascular ischaemia[8]. Hence some investigators focused on patients with features of microvascular ischaemia, whereas other focused on those with an impaired coronary vasodilator reserve (original Microvascular Angina). Although the two entities may coexist, the interrelationship requires further investigation.
Microvascular spasm
This term was coined by Mohri et al.[9], who observed chest pain and ischaemic ECG changes in patients during acetylcholine (ACh) provocation testing, despite the absence of epicardial coronary artery spasm. The presence of ischaemia was further confirmed by transcardiac lactate measurements. Thus the ACh-induced myocardial ischaemia in the absence of epicardial coronary spasm was attributed to microvascular spasm. This approach provides a pragmatic diagnostic strategy in the diagnosis of CMD since the ACh provocation test both diagnoses microvascular spasm and excludes the presence of inducible epicardial coronary spasms.
Coronary slow flow phenomenon
Initially described by Tambe et al.[10], this angiographic phenomenon is defined as a delayed passage of contrast medium through the coronary arterial tree, despite the absence of obstructive coronary arteries. It has been clinically characterised[11], and the underlying pathophysiology is confirmed as an increased resting coronary microvascular resistance[12]. Hence this disorder differs from other coronary microvascular disorders since the abnormal coronary vasomotor disorder is evident at rest.
Characteristics and prognosis of CMD
Over three-quarters of patients with suspected ischaemia and no obstructive coronary artery disease have identifiable coronary vasomotor disorders[13]. Patients with CMD are younger at the time of diagnosis (~49 years) and more often female (up to 70%)[14] compared to those with obstructive CAD. This has led to speculation that women have a predilection to non-obstructive coronary arteries, whereas men are more likely to have atherosclerotic obstructive coronary artery disease[1]. In women with suspected ischaemia and no obstructive coronary artery disease, a higher baseline average peak velocity (bAPV) is associated with greater angina severity by the higher use of anti-angina medication, suggesting that perhaps a high bAPV contributes to impaired CFR and may represent a specific pathophysiological contributor to CMD[15]. The long-term prognosis of patients with angina in the absence of obstructive coronary artery disease is heterogeneous, with a systematic review and meta-analysis suggesting that the presence of mild atherosclerosis or evidence of myocardial ischaemia may impact prognosis[16]. Moreover, in women with evidence of ischaemia and non-obstructive coronary arteries, impaired microvascular vasodilatory response to adenosine was predictive of major adverse cardiac events (MACE = cardiovascular death, myocardial infarction, stroke, and heart failure) during a median follow-up of 9.7 years[17].
COVADIS definition
The Coronary Vasomotion Disorders International Study Group (COVADIS) have recently proposed all-encompassing clinical criteria for patients with primary coronary microvascular disorders, including the following attributes: (1) ischaemic symptoms; (2) objective evidence of myocardial ischaemia; (3) absence of obstructive CAD; and (4) evidence of CMD (as demonstrated by impaired CFR, microvascular spasm, abnormal iMR/hMR, or the coronary slow flow phenomenon)[18]. Patients fulfilling all these criteria are considered as having “Definitive Microvascular Angina”, whereas those with only 3 criteria are considered as “Suspected Microvascular Angina”[18]. Accordingly, they expanded the clinical context of the term “Microvascular Angina” compared to the original term.
MYOCARDIAL ISCHAEMIA AS A PATHOPHYSIOLOGICAL MECHANISM
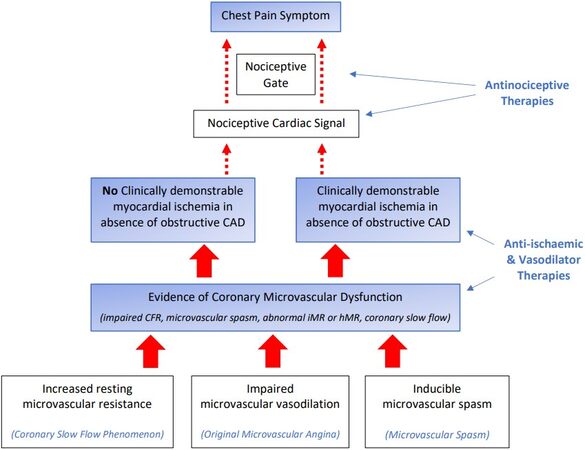
Based upon the above COVADIS definition, patients with microvascular angina require two essential pathophysiological elements, namely evidence of myocardial ischaemia and CMD, to account for the chest pain experienced by these patients in the absence of obstructive CAD. The presence of both of these pathophysiological elements provides evidence that the chest pain is cardiac in origin and excludes non-cardiac causes, despite some clinicians labelling the patients with “non-cardiac chest pain”. These pathophysiological elements also provide a logical explanation as to the mechanisms responsible for the chest pain, with CMD producing myocardial ischaemia [Figure 1] and analogous to the paradigm of obstructive CAD producing myocardial ischaemia. Furthermore, as shown in Figure 1, the previously characterised coronary microvascular disorder endotypes may produce CMD via different mechanisms, but all have a final common pathway of myocardial ischaemia producing chest pain.
Figure 1. Pathophysiological mechanisms of coronary microvascular disorders in relation to clinical manifestations and therapeutic targets. Pathophysiologic mechanisms in clear boxes. Clinical Manifestations (COVADIS Diagnostic Criteria) in blue shaded boxes. Therapeutic Strategies in blue free text. CFR: Coronary flow reserve; iMR: index of Microvascular resistance; hMR: hyperaemic microvascular resistance.
CMD mechanisms producing myocardial ischaemia
This requires an understanding of the regulation of coronary microcirculation. Beyond the epicardial coronary arteries, which largely serve as conductance and capacitance vessels, commences the coronary microcirculation, including vessels < 500 microns in diameter. The microcirculation comprises of pre-arterioles (100-500 mcm), arterioles (100 mcm), capillaries (10 mcm) and venules. The pre-arterioles and arterioles are the resistance vessels within the coronary circulation and thus exert the greatest impact on coronary blood flow. In contrast, the capillaries are responsible for gas/nutrient exchange with myocardial cells, and the venules drain to the coronary sinus. The regulation of coronary microvascular resistance is complex, being influenced by extravascular compressive forces, perfusion pressure, and coronary autoregulation, as well as neurohumoral, endothelial, and metabolic factors. Moreover, the coronary microvascular resistance regulation is not distributed uniformly across the myocardium but varies across different vascular segments and microdomains. This variability in myocardial perfusion is controlled by both the pre-arterioles and arterioles, which differ in their functions. The pre-arterioles are not only influenced by local vascular factors but also by extravascular neurohumoral stimuli (e.g., adrenaline). These stimuli may influence myocardial perfusion within a local vascular territory by adjusting pre-arteriolar vascular tone. In contrast, arterioles have a pivotal role in coronary autoregulation. This process involves maintaining consistent perfusion within a localised microdomain, despite a wide range of changing driving perfusion pressures. The exact mechanisms involved in this pressure-flow autoregulation are unclear, but vascular myogenic tone (i.e., vascular smooth muscle ability to constrict in response to increased perfusion pressure) is thought to play a role.
The coronary microvascular disorders [Table 1] exhibit disturbed coronary microvascular resistance, which may manifest as an increased resting microvascular resistance, impaired microvascular vasodilation, and inducible microvascular spasm. However, the disturbances in the microvascular regulatory pathways responsible for these vasomotor perturbations remain to be fully elucidated.
Increased microvascular resistance
Since its first description of 6 cases in 1972[10], the delayed passage of angiographic contrast in the coronary slow flow phenomenon (CSFP) has been attributed to increased microvascular resistance. This has been supported by coronary haemodynamic studies demonstrating elevated resting microvascular resistance but an intact vasodilatory capacity (i.e., coronary flow reserve)[12,19]. The cause of this increased resting microvascular resistance requires further elucidation but may involve structural or functional abnormalities. Mosseri et al.[20] undertook cardiac biopsies in patients with the CSFP and demonstrated abnormal small vessels and capillaries, with endothelial cell swelling and common degeneration findings, which may potentially structurally obstruct the vessel lumen. Capillary rarefaction is another structural cause of an increased resting resistance, although it was not evaluated in the biopsy studies. In relation to functional abnormalities, increased intramyocardial compressive forces and microvascular constriction are possible causes, although the former has not been evaluated in the CSFP. Potential autacoids that may mediate the increased microvascular resistance include neuropeptide Y, endothelin-1, and thromboxane A2[12]. Evidence supporting a pathogenetic role for neuropeptide Y and endothelin-1 include the induction of the CSFP by respective intracoronary infusion of these vasoconstrictors in humans[21] and animals[22,23] models. Also, increased plasma levels of endothelin-1[24] and thromboxane A2[25] have been reported in patients with the CSFP.
Impaired microvascular dilation
In response to increased oxygen demand, autacoids are released that have an autoregulatory function and dilate the arteriolar circulation, resulting in an increased coronary blood flow. With a maximal hyperaemic stimulus (e.g., adenosine or dipyridamole), the resting coronary blood flow should at least double from the resting state, i.e., the coronary flow reserve (maximal hyperaemic coronary blood flow/resting flow) > 2. If inadequate vasodilation occurs in response to the hyperaemic stimulus, then it infers a disturbance in coronary blood flow regulation. This may be multifactorial, including an increased resting coronary resistance with inadequate compensatory vasodilatory reserve or a reduced capacity to vasodilate. The mechanisms responsible for the disturbed coronary blood flow regulation require further investigation, but microvascular endothelial dysfunction appears to play a role. Egashira et al.[26], measured coronary blood flow as a marker of coronary microvascular function and demonstrated that endothelium-dependent microvascular vasodilation was impaired in patients with microvascular ischaemia whereas endothelium-independent vasodilation was intact. Whether coronary risk factors, which are key determinants in large vessel endothelium-dependent vasodilation, are important in microvascular endothelium-dependent vasodilation requires further investigation.
Inducible microvascular spasm
The mechanism responsible for the microvascular hyper-reactivity to ACh stimuli also requires investigation. Since ACh is an endothelium-dependent vasodilator, an endothelium-dependent mechanism may be inferred. However, the dose of ACh used in the provocative spasm testing is 5-10 fold greater than the doses used for endothelial function testing and will have a direct effect on the vascular smooth muscle beyond the endothelium. Hence the underlying mechanism appears to be a microvascular smooth muscle cell hyper-reactivity to Ach, similar to that observed in vasospastic angina.
ABNORMAL NOCICEPTION AS A PATHOPHYSIOLOGICAL MECHANISM
The concept that CMD (via a variety of mechanisms) produces myocardial ischaemia, which initiates a neural pain pathway so that the ischaemia is perceived as chest pain by the sensory cortex in patients with microvascular angina [Figure 1], has been questioned by some researchers. Puzzling observations in patients with microvascular angina, which are difficult to explain using this paradigm include: (1) not all patients with evidence of CMD have evidence of myocardial ischaemia[7]; (2) patients with apparent microvascular ischaemia (as suggested by a positive stress ECG) do not exhibit classical metabolic markers (e.g., lactate production) of myocardial ischaemia during rapid atrial pacing[27]; and (3) unlike obstructive coronary artery disease, where ischaemia is associated with a transient regional wall motion abnormality (e.g., a positive stress echocardiogram), patients with microvascular ischaemia have preserved systolic function during myocardial ischaemia[28], prompting speculation that there is a different “ischaemic cascade” in CMD. Accordingly, the “ischaemic paradigm” has been challenged in microvascular angina[29].
In addition to these discrepancies between CMD, myocardial ischaemia, and chest pain in patients with coronary microvascular disorders, multiple studies have demonstrated an abnormal pain perception [Table 2]. This has fostered the second school of thought in the clinico-pathophysiology mechanisms of coronary microvascular disorders, beyond the “myocardial ischaemia hypothesis” to an “abnormal nociception hypothesis”.
Abnormal pain perception in patients with coronary microvascular disorders
| Abnormal pain perception | Supporting studies |
| Increased sensitivity to cardiac stimuli | · Intracardiac catheter manipulation provoked chest pain[35] · Direct cardiac stimulation with pacing wire[36,37] · Increased perception of pacing-induced pain (even during sham stimulation periods[38] |
| Abnormal cortical pain processing | · Impaired habituation to pain stimuli[39] · Functional neuroimaging during high dose dobutamine infusion demonstrated greater right anterior insular activity[30] |
| Lower cardiac pain threshold | · Low pain threshold and low tolerance to pain induced by adenosine[40] and epinephrine[41] infusion |
| Positive response to imipramine | · Imipramine (an anti-depressant used for chronic pain syndromes) was an effective anti-anginal agent in microvascular angina patients[42] |
In a landmark controlled study, Rosen et al.[30] administered a high dose dobutamine infusion to patients with apparent microvascular ischaemia, CAD, and control patients, assessing regional cerebral blood flow via PET as a marker of cerebral activity. Compared with the other groups, they observed more extensive and enhanced cortical activation in the patients with microvascular ischaemia (especially in the right insula). This suggests that the right insula (which receives the most cardiopulmonary sensory input) has a significant role in the increased pain perception in patients with microvascular ischaemia.
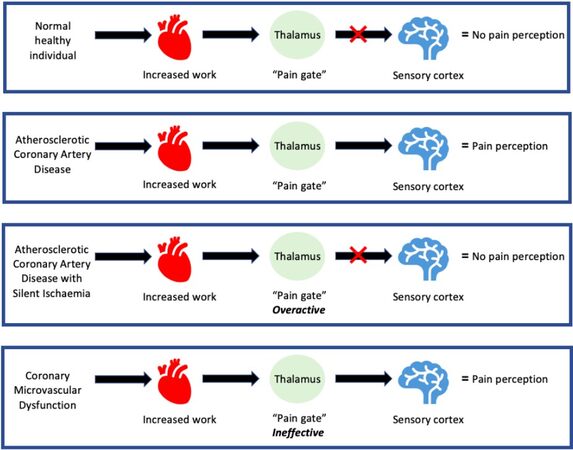
The “gate theory”[30] of pain perception has been proposed to explain these findings. With increased cardiac work, a normal healthy individual will have a continuous stream of afferent stimuli from the heart, which reaches the thalamus, but the signals do not reach the cortex; hence there is no pain perception. In myocardial ischaemia in CAD patients, secondary to increased cardiac work, the stream of afferent stimuli is stronger and overcomes the filtering ability of the thalamus, which will therefore allow pain signals to reach the cortex; thus, there is a perception of pain by the patient. However, patients with CAD who experience silent myocardial ischaemia may have altered handling of afferent signals from the heart at the central level (“overactive gate”), which contributes to a lack of perception of chest pain. On the contrary, patients with apparent microvascular ischaemia may have an ineffective thalamic “gate” that would allow inadequate cortical activation by afferent stimuli from the heart, thus causing increased pain perception
As summarised in Figure 1, two “schools of thought” may be considered in the approach to coronary microvascular disorders. The conventional approach where coronary microvascular dysfunction produces myocardial ischaemia, and in turn, angina is represented in the right-sided pathway. The left-sided pathway [Figure 1] reflects the abnormal nociception pathway, where myocardial ischaemia is not overtly responsible for the perceived pain, and abnormal pain perception is primarily responsible for the symptoms. The role of myocardial ischaemia in this pathway requires further clarification since it may be absent, and thus the abnormal pain perception may directly arise from the coronary microvascular dysfunction. Alternatively, subclinical myocardial ischaemia may be present, which would not give rise to symptoms that are exaggerated by the abnormal pain perception. Consideration should also be given to the myocardial ischaemia being present (and responsible for the chest pain) but beyond the detection of contemporary diagnostic techniques.
THERAPEUTIC IMPLICATIONS
Understanding the pathophysiological mechanisms responsible for coronary microvascular disorders is not merely a theoretical academic exercise since the underlying mechanisms will be the targets for therapeutic strategies. Thus, if the clinical assessment implicates myocardial ischaemia as the cause of chest pain, anti-ischaemic therapies such as beta-blockers, calcium channel blockers, and ranolazine should be considered. However, if an abnormal pain perception is believed responsible for the symptoms, anti-nociceptive therapies such as antidepressants, methylxanthines, and neurostimulators should be considered. While clinical assessment of the patient may predicate one strategy over the other, the pragmatic approach adopted by many clinicians is to first utilise conventional anti-ischaemic medications and progress on to anti-nociceptive therapeutic strategies if unresponsive.
CONCLUDING THOUGHTS
Coronary microvascular dysfunction patients represent a heterogenous group with underlying mechanisms implicating both coronary vasculature dysfunction and altered nociceptive perception. The application of invasive or non-invasive methods for the diagnosis of CMD depends on patient characteristics and preference, clinical presentation as well as local experience, and availability of the respective method. A personalised approach for treatment should be carried out, targeting the heart or the brain depending on the underlying pathogenesis responsible for chest pain in CMD patients.
DECLARATIONS
Author’s contributionsConducted a review of the literature and prepared the manuscript draft: La S, Tavella R, Pasupathy S, Beltrame JF
Substantially involved in the conception, drafting, and editing of the manuscript: La S, Tavella R,
Final approval of the manuscript: La S, Tavella R, Pasupathy S, Beltrame JF
Availability of data and materialsNot applicable.
Financial support and sponsorshipLa S is supported by The Australian Government Research Training Program Stipend.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886-95.
2. Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009;18:19-27.
3. Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med 1967;276:1063-6.
4. Arbogast R, Bourassa MG. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms. Am J Cardiol 1973;32:257-63.
5. Kemp HG. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol 1973;32:375-6.
6. Cannon RO, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol 1988;61:1338-43.
7. Cannon RO, Watson RM, Rosing DR, Epstein SE. Angina caused by reduced vasodilator reserve of the small coronary arteries. J Am Coll Cardiol 1983;1:1359-73.
8. Maseri A, Crea F, Kaski JC, Crake T. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol 1991;17:499-506.
9. Mohri M, Koyanagi M, Egashira K, et al. Angina pectoris caused by coronary microvascular spasm. Lancet 1998;351:1165-9.
10. Tambe A, Demany M, Zimmerman HA, Mascarenhas E. Angina pectoris and slow flow velocity of dye in coronary arteries - a new angiographic finding. Am Heart J 1972;84:66-71.
11. Beltrame JF, Limaye SB, Horowitz JD. The coronary slow flow phenomenon - a new coronary microvascular disorder. Cardiology 2002;97:197-202.
12. Beltrame JF, Limaye SB, Wuttke RD, Horowitz JD. Coronary hemodynamic and metabolic studies of the coronary slow flow phenomenon. Am Heart J 2003;146:84-90.
13. Ford TJ, Yii E, Sidik N, et al. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovasc Interv 2019;12:e008126.
14. Dean J, Cruz SD, Mehta PK, Merz CN. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nat Rev Cardiol 2015;12:406-14.
15. Suppogu N, Wei J, Quesada O, et al. Angina relates to coronary flow in women with ischemia and no obstructive coronary artery disease. Int J Cardiol 2021;333:35-9.
16. Radico F, Zimarino M, Fulgenzi F, et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J 2018;39:2135-46.
17. AlBadri A, Bairey Merz CN, Johnson BD, et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol 2019;73:684-93.
18. Ong P, Camici PG, Beltrame JF, et al. Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16-20.
19. Leone MC, Gori T, Fineschi M. The coronary slow flow phenomenon: a new cardiac "Y" syndrome? Clin Hemorheol Microcirc 2008;39:185-90.
20. Mosseri M, Yarom R, Gotsman MS, Hasin Y. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation 1986;74:964-72.
21. Kaksi J, Tousoulis D, Rosano G, Clarke J, Davies G. Role of neuropeptide Y in the pathogenesis of syndrome X. Eur Heart J 1992;13:103.
22. Larkin SW, Clarke JG, Keogh BE, et al. Intracoronary endothelin induces myocardial ischemia by small vessel constriction in the dog. Am J Cardiol 1989;64:956-8.
23. Hirata K, Matsuda Y, Akita H, Yokoyama M, Fukuzaki H. Myocardial ischaemia induced by endothelin in the intact rabbit: angiographic analysis. Cardiovasc Res 1990;24:879-83.
24. Pekdemir H, Polat G, Cin VG, et al. Elevated plasma endothelin-1 levels in coronary sinus during rapid right atrial pacing in patients with slow coronary flow. Int J Cardiol 2004;97:35-41.
25. Donato M, Fantini F, Maioli M, Prisco D, Rogasi PG, Neri Serneri GG. Blood velocity in the coronary artery circulation: relation to thromboxane A2 levels in coronary sinus in patients with angiographically normal coronary arteries. Cathet Cardiovasc Diagn 1987;13:162-6.
26. Egashira K, Inou T, Hirooka Y, Yamada A, Urabe Y, Takeshita A. Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. N Engl J Med 1993;328:1659-64.
27. Camici PG, Marraccini P, Lorenzoni R, et al. Coronary hemodynamics and myocardial metabolism in patients with syndrome X: response to pacing stress. J Am Coll Cardiol 1991;17:1461-70.
28. Picano E, Lattanzi F, Masini M, Distante A, L'abbate A. Usefulness of a high-dose dipyridamole-echocardiography test for diagnosis of syndrome X. Am J Cardiol 1987;60:508-12.
29. Picano E. The alternative "ischemic" cascade in coronary microvascular disease. Cardiologia 1999;44:791-5.
30. Rosen SD, Paulesu E, Wise RJ, Camici PG. Central neural contribution to the perception of chest pain in cardiac syndrome X. Heart 2002;87:513-9.
31. Rosen SD, Paulesu E, Nihoyannopoulos P, et al. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med 1996;124:939-49.
32. Crea F, Lanza GA. Angina pectoris and normal coronary arteries: cardiac syndrome X. Heart 2004;90:457-63.
33. Epstein SE, Cannon RO, 3rd, Bonow RO. Exercise testing in patients with microvascular angina. Circulation 1991;83:III73-6.
35. Shapiro LM, Crake T, Poole-Wilson PA. Is altered cardiac sensation responsible for chest pain in patients with normal coronary arteries? Br Med J (Clin Res Ed) 1988;296:170-1.
36. Cannon RO, Quyyumi AA, Schenke WH, et al. Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries. J Am Coll Cardiol 1990;16:1359-66.
37. Chauhan A, Mullins PA, Thuraisingham SI, Taylor G, Petch MC, Schofield PM. Abnormal cardiac pain perception in syndrome X. J Am Coll Cardiol 1994;24:329-35.
38. Pasceri V, Lanza GA, Buffon A, Montenero AS, Crea F, Maseri A. Role of Abnormal Pain Sensitivity and Behavioral Factors in Determining Chest Pain in Syndrome X. J Am Coll Cardiol 1998;31:62-6.
39. Valeriani M, Sestito A, Le Pera D, et al. Abnormal cortical pain processing in patients with cardiac syndrome X. Eur Heart J 2005;26:975-82.
40. Lagerqvist B, Sylvén C, Waldenström A. Lower threshold for adenosine-induced chest pain in patients with angina and normal coronary angiograms. Br Heart J 1992;68:282-5.
41. Eriksson B, Svedenhag J, Martinsson A, Sylvén C. Effect of epinephrine infusion on chest pain in syndrome X in the absence of signs of myocardial ischemia. Am J Cardiol 1995;75:241-5.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
La S, Tavella R, Pasupathy S, Beltrame JF. Clinico-pathophysiological considerations in coronary microvascular disorders. Vessel Plus 2022;6:1. http://dx.doi.org/10.20517/2574-1209.2021.68
AMA Style
La S, Tavella R, Pasupathy S, Beltrame JF. Clinico-pathophysiological considerations in coronary microvascular disorders. Vessel Plus. 2022; 6: 1. http://dx.doi.org/10.20517/2574-1209.2021.68
Chicago/Turabian Style
La, Sarena, Rosanna Tavella, Sivabaskari Pasupathy, John F. Beltrame. 2022. "Clinico-pathophysiological considerations in coronary microvascular disorders" Vessel Plus. 6: 1. http://dx.doi.org/10.20517/2574-1209.2021.68
ACS Style
La, S.; Tavella R.; Pasupathy S.; Beltrame JF. Clinico-pathophysiological considerations in coronary microvascular disorders. Vessel Plus. 2022, 6, 1. http://dx.doi.org/10.20517/2574-1209.2021.68
About This Article
Copyright
Data & Comments
Data

 Cite This Article 7 clicks
Cite This Article 7 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.