Histological analysis of thrombi retrieved after acute ischemic stroke from large vessel occlusion: from research to clinical practice
Abstract
Emergent reperfusion therapies have improved acute ischemic stroke prognosis, but many patients are still bound to bad clinical outcome, probably because of our incomplete knowledge of its pathophysiology. Thanks to mechanical thrombectomy, occluding material is available for histological analysis. Several studies investigated the possible relationship between thrombus composition and clinical, procedural, and radiological variables of acute ischemic stroke. The potential value of thrombus analysis as a tool for clinical practice and research is still not defined, as data from the literature are heterogeneous and sometimes conflicting. We propose a review of the existing literature regarding histological analysis of thrombi in acute ischemic stroke. We classified articles on clot composition according to the clinical variable explored in each study. We first distinguished articles about etiology, procedural, and radiological variables, and then we performed a subclassification for each group. This review could help both in the interpretation of thrombus analysis in clinical practice and in its usage for future research.
Keywords
INTRODUCTION
Mechanical thrombectomy (MT) represents a milestone in the field of acute ischemic stroke (AIS) therapy thanks to its demonstrated beneficial effect over a large portion of patients, who were otherwise bound to high rates of poor functional outcome[1].Nonetheless, AIS remains a leading cause of disability and the second cause of death worldwide[2]. About 30% of technically successful MTs are classified as futile recanalization, i.e., complete reperfusion without good clinical outcome[3]. The reasons for clinically unsuccessful therapeutic interventions on AIS are not totally understood, probably because of our imperfect knowledge of AIS pathophysiology.
Besides their therapeutic effect, endovascular treatments made it possible to analyze thrombotic material responsible for large vessels occlusion. Although in the past years several researchers focused their work on the study of clots composition in AIS, we are far from a routine usage of thrombus histology analysis in clinical practice, as both the required analysis and their relationship with clinical and radiological variables are not standardized[4]. However, the existing evidence outlines that cerebral artery occlusion is a multifactorial process which involves the source of the occluding material, the site of occlusion, comorbidities, inflammatory state, and even the recanalization procedure itself[5].
The aim of this review is to provide an overview of the present status of the analysis of thrombi from occluded intracerebral arteries, with its possible implications in terms of research and clinical practice.
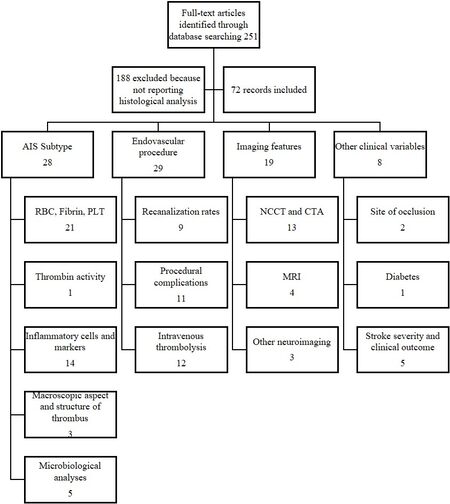
We conducted a systematic search on PubMed and Ovid MEDLINE online databases with the following keywords: stroke, thrombus, clot, histology, and thrombectomy. Among the results of our research, we excluded studies in which histological analysis was not performed. The selected articles were classified according to the relationship between histology and stroke etiology, procedural variables, or radiological characteristics. Articles that do not fit any of the abovementioned categories are collected in the paragraph about the relationship between thrombus histology and other clinical variables.
We distinguished different subgroups for each main group. The literature about AIS etiology is divided according to the histological analysis performed in each study: red blood cells, fibrin and platelet content, thrombin activity, inflammatory cells and markers, macroscopic aspect and structure of the thrombus, and microbiological analysis. As for endovascular procedures, we separate the studies into those discussing recanalization rates and those regarding procedural complication. Articles exploring correlation between clots and neuroimaging are classified depending on the neuroimaging technique used by researchers [Figure 1].
HISTOLOGICAL ANALYSIS OF THROMBUS AND ACUTE ISCHEMIC STROKE ETIOLOGICAL SUBTYPE
As stroke investigators started studying thrombus composition, the scientific community provided recommendations on how to perform the histological analysis[6]. According to the international study group, quantification of red blood cells (RBCs) and fibrin should always be included, and it is also recommended to look for platelets (PLT), white blood cells (WBCs), and von Willebrand factor (VWF)[6]. Based on our literature, studies including the main composition of clots, mainly RBCs, PLT, and fibrin, comprised the largest group, with a total of 33 clinical studies.
Red blood cells, fibrin, and platelets content in thrombus
In 21 articles, researchers attempted to find a correlation between RBC, PLT, and fibrin contents and stroke subtypes. According to seven studies, thrombi retrieved from cardioembolic (CE) strokes showed significantly lower RBC content or higher fibrin content and platelet count compared to other etiologies[7-14]; two studies collected data from more than 100 patients[8,9]. Interestingly, one study compared histological analysis of retrieved thrombi with that of atherosclerotic plaques and found high RBC content in both when specimens were retrieved from patients with AIS from large artery atherothrombosis (LAA). The same study also found that specimens from valvular heart disease and those retrieved from patients with CE stroke both had a significantly higher fibrin content as compared to thrombi from other etiologies[10].
Khismatullin et al.[14] studied the morphology of clot components in addition to the quantitative analysis and found that CE thrombi, compared to those from LAA, more frequently contained enlarged platelets (named balloon-like platelets), which are believed to be a sign of platelet activation. Furthermore, the distribution of components seems to be different in the various stroke subtypes: platelets organized in peripheral patterns in LAA strokes, while they are in clustered patterns in CE ones[15].
Three studies reported that CE events and cryptogenic strokes (CS) have a similar thrombus composition[7,9,10], suggesting that thrombus histology could help redefine stroke etiology in CS patients. This might be useful, considering that CS accounts for about 30% of all AIS and is still a major unresolved issue as regards secondary prevention therapy.
Contrary to the abovementioned studies, one large study on 105 patients found a higher percentage of platelet content in strokes from LAA compared to those from CE source; moreover, clots from CS were found to be similar to the ones from the LAA group[16]. In line with these findings, in another study on a smaller cohort of 37 patients, higher RBC and lower fibrin content clots were found in CE strokes rather than in other stroke subtypes[17]. Although the results from Fitzgerald et al.[16] might seem in contrast with previous ones, the authors commented that in their analysis platelets were counted separately from fibrin, while other protocols considered them together.
Recently, an attempt to create a predictive scale for stroke etiology has been done, by combining the type of occlusion (branching site vs. truncal type), pattern of platelet distribution in thrombi, and entity of carotid atherosclerosis in a four-point scale. After the validation of the scale named BOCS2, scores > 2 had a high sensitivity (93.5%) and specificity (100%) in predicting CE strokes[15].
Hence, more data from larger cohorts of patients and more uniformity among histological analysis protocols are needed, to better compare literature data and finally use clot histology for ethiopatogenic classification in clinical practice. Importantly, available data highlight how the classic concept of “white” and “red” thrombi, attributed, respectively, to atherothrombotic and cardioembolic strokes[18], needs to be reconsidered in a multifactorial disease as AIS.
Thrombin activity in thrombus
Looking for alternative methods to determine occluding clot origin, one study analyzed thrombin activity trends in thrombi retrieved from patients divided into 5 groups according to etiology of AIS. Thrombin activity was measured by a fluorometric assay quantifying the cleavage of the synthetic peptide substrate, after serial washes of thrombotic material[19]. Researchers found a significant difference between patterns from patients with atrial fibrillation and those with LAA stroke, respectively, with decreasing and steady trend of thrombin activity. Interestingly, no difference was found in terms of RBC and platelet content. The authors suggested that thrombi from low-flow cardiac chambers would still be rich in thrombin after retrieval, while this is readily washed out during in vitro processing. On the contrary, LAA thrombi would be intensively washed out by high arterial flow on plaques; consequently, after retrieval, their thrombin activity remains steady during in vitro processing. Although this was a pilot study with a small sample of patients, it gave innovative insight into clot analysis.
Inflammatory cells and markers in thrombus
Cerebral arterial thrombosis is a complex process which involves inflammation cascade activation immediately after vessel occlusion, contributing through different pathways to both brain damage and protection[20]. Clot analysis therefore revealed the presence of inflammatory cells and mediators in their composition; this was already evident in one study on MT that showed prevalent T-cell and monocyte contents in red thrombi and higher VWF in white ones[21]. Ten studies looked for a relationship between WBCs detected in retrieved thrombi and stroke subtypes[9-13,16,17,22-24]. Boeckh-Behrens et al.[9,23],
First identified for their role in the innate immune response[26], neutrophil extracellular traps (NETs) are made up of fibrin networks of extracellular DNA born from the association of decondensed chromatin, histones, and granule proteins released by neutrophil[27]. NETs’ role was first investigated in both arterial and venous thrombosis from different body districts. A study on AIS identified NETs as prominent extracellular nucleic acid-rich areas on thrombi stained with hematoxylin and eosin and immunostained for citrullinated histone H, which is a marker of NETs. Investigators found that both neutrophils and NETs were common in specimens; moreover, NET content was almost doubled in clots from CE origin[27]. Savchenko et al.[28] assumed that NETs are involved in thrombus formation in the condition of stasis, in agreement with the previous evidence of NETs in venous thrombi. Involvement of NETs in AIS was also confirmed recently by one study comparing AIS thrombi with clots retrieved after acute myocardial infarction, which identified NETs in all stroke cases but only in 20% of the coronary artery occlusions[25]. Moreover, clots from LAA had lower numbers of NETs than those of CE and unknown etiology strokes[25]. The contrasting results from different studies on thrombus histology might be in part a consequence of the semiquantitative methods used to define thrombus composition. The quantification of thrombus content through specific assays may represent a more accurate analysis. A French group used specific assays for heme, DNA, and glycoprotein VI to quantify, respectively, RBCs, WBCs, and platelets in clots from AIS patients. Their results show how CE cases were richer in DNA, which in thrombi derives mostly from WBCs or possibly NETs[29]. Moreover, the same study highlighted a potential diagnostic value of DNA content quantification and DNA/GPVI ratio in thrombi for the differentiation of AIS etiological subtypes[29].
One case report about thrombus histological analysis in a patient with antiphospholipid syndrome is worth being mentioned. The described patient was diagnosed as having Libman-Sacks embolic stroke. The thrombus showed acellular homogeneous eosinophilic hyalinized collagenous tissue and widespread staining for IgG, C1q, C3, and IgA, while bacteriological and fungal staining resulted negative[30]. This represents an interesting example of the diagnostic usage of retrieved thrombi in AIS from rare etiologies.
Macroscopic aspect and structure of thrombus
Two studies found an association between clot macroscopic characteristics and stroke etiology. In the first one analyzing sample colors, white thrombi were found to relate with atypical stroke etiology[31]. In the second study, researchers measured clot area and found LAA thrombi to be significantly larger and richer in RBCs as compared to other etiologies[13].
Focusing on fibrin structure, a recent study reported the major prevalence of the fibrillar type of fibrin rather than fibrin sponge in LAA thrombi, while in CE cases mostly fibrin bundles were found[14]. In particular, according to the age-dependent staining of fibrin[32], the fibrin from CE clots was mainly of the “young type”[14]. Conversely, another small study classified thrombi according to fibrin structure and did not find any significant association of thrombus organization with stroke subtypes[33].
Microbiological analyses of thrombus
Four articles focused on bacterial detection in thrombi retrieved after mechanical thrombectomy[34-37]. In the largest series, clot were systematically studied including Gram and Gomori thrichrome staining; bacteria were detected in four of 65 specimens, in two of which endocarditis was evident[35]. A small case series[34] and one case report[36] on patients with diagnosed endocarditis showed bacterial detection in thrombi, suggesting that it might help in the choice of antibiotic therapy. Despite the scarce data, it is advisable to include bacterial staining in the histological analysis of thrombi.
Inflammatory state and bacteremia contribute to increase stroke risk[38,39]. On this basis, a study searched bacterial DNA on clots retrieved after MT. The result was that 84% of the analyzed thrombi contained bacterial DNA, especially from Streptococcus mitis, typically found in the oral cavity[40]. Actually, these data do not provide any causative link between oral bacterial infections and AIS but stress the role of chronic infections and inflammation in enhancing thrombosis in AIS.
CORRELATION BETWEEN THROMBUS HISTOLOGY AND ENDOVASCULAR PROCEDURE
Recanalization rates
As to procedural features, studies focusing on thrombus composition outlined mainly its relationship with procedural times, number of passages, recanalization rates, and possible periprocedural complication.
Successful reperfusion, defined as a final Thrombolysis in Cerebral Infarction (TICI)[41] score 2b-3 after mechanical thrombectomy is an established prognostic factor for patients with AIS from LVO. Most studies focusing on procedural success report higher rates of complete recanalization in the case of occlusions due to RBC thrombi and lower rates of complete recanalization when platelets and fibrin (FP) are the main components[16,42-44]. Retrieval of platelet-rich thrombi might be troublesome because of their friction properties[45] and stiffness, as an in vitro model showed that platelets provoke fibrin network remodeling leading to higher clot density[46]. Moreover, platelet-rich areas in thrombi include complex and dense structures of fibrin packed with DNA and VWF, as compared to RBC-rich areas which have a thin fibrin structure[47]. Lower recanalization rates might be the direct consequence of the longer and more complex procedure during the retrieval of FP-rich clots.
One study on clot analogs showed that stent retrievers required more time to reach greater indentation depth in these clots (the majority of clot integration occurring after 3 min)[48]. Weafer et al.[48] showed that strut indentation depends on the ratio between fibrin and cells in the thrombus; when the former is prevalent, stent retrievers catch clots through fibrin stretching, while, in RBC-rich thrombi, the main mechanism of action of stent retrievers is fibrin rupture.
It should be recalled that all data about thrombus composition and recanalization are unavoidably incomplete, as the composition of thrombi from failed procedures (TICI 0-1), which means not retrieved clots, is unknown.
Most frequently, RBC-rich thrombi were reported to be retrieved in shorter duration procedures requiring fewer passages[8,49,50], while fibrin-rich clots seem to be related to more passages and therefore longer times for removal. This would be in agreement with in vitro studies outlining the greater stiffness of fibrin-rich thrombi[51]. Moreover, it has been reported that fibrin content relates to higher clot friction properties on experimental surfaces and animal vessel walls[45]. One study on cadavers reported how aspiration devices need more passes to achieve complete ingestion of fibrin-rich clots[52]. Hence, the identification in emergency imaging of this kind of thrombi (see below) could help to choose the best devices for the procedures and the shorten time of recanalization.
One study including 108 AIS patients found that higher content of NETs in clots correlated significantly with longer procedure and the need for more passes before recanalization[53]. Moreover, ex vivo experiments from this latter study targeting NETs through recombinant DNAse 1 administration showed an accelerated r-TPA-mediated thrombus dissolution[53]. NETs, thanks to their DNA and histone contents, might modify fibrin structure of occluding material in thrombotic processes, making it more resistant to pharmacological and mechanical retrieval[54]. This latter evidence makes NETs a potential therapeutic target for future studies.
Procedural complications
Thrombus behavior during reperfusion therapies seems to depend on its composition. In particular, researchers reported how a higher percentage of RBCs and a shorter length at histological analysis are consistent with thrombus migration before mechanical thrombectomy[55-57], i.e., a more distal clot location at digital subtraction angiography as compared to location on emergent CT or CTA. Clot migration could be the consequence of lower friction properties of RBC-rich thrombi[45].
The choice of the correct device before endovascular procedure is highly important and should theoretically consider thrombus properties. Secondary embolism (SE) is a possible complication of mechanical thrombectomy, which implies fragmentation of the thrombus with occlusion of distal branches, hardly reachable with endovascular devices, and lower reperfusion rates. According to one study, a higher percentage of fibrin and a lower one of RBCs in clots are significantly associated with SE[8]. On the contrary, a more recent study found RBC-rich clots to be correlated with SE[58], and a similar trend had been reported in a previous study[59]. Although the authors claimed that the different histological techniques might influence these contrasting results, it should be considered as well that, while in the series from
Although very effective, MT is an invasive procedure that can be traumatic for the cerebral vessel wall. Endovascular procedure traumatism has been proved by the presence in specimens of CD34-positive endothelial cells[63] or banded collagen fibers with distinct boundary existing at the margin or outside of the thrombus[64].
In a study on a small cohort of patients, CD34-positive cells were found in 20 out of 48 patients, but in only seven cases cells were organized in cluster and the analysis did not show any evidence of intimal damage, such as the presence of subendothelial connective tissue in thrombi[63]. Given their findings, investigators concluded that stent retrieving does not cause relevant intimal damage, and endothelial cells found in thrombi might be the hint of the physiological turnover of endothelium or of an initial organization of the thrombus. On the other hand, in a larger series, vessel wall components were found, with a different histological technique, in 16% of analyzed specimens; moreover, parts of internal elastic lamina were present in 12 out of 150 thrombi, suggesting an involvement of the tunica media by MT-induced vascular injury[64]. These contrasting results require confirmation by other studies with standardized histological analysis. Interestingly, the presence of vessel wall components in thrombi was associated with a higher number of passages, more distal location of the occlusion (specifically M2 and P2), and low content of RBCs[64], suggesting that more complex procedures might cause wall damage.
Analysis of thrombi in patients treated with intravenous thrombolysis
Recombinant tissue plasminogen activator (rtPA) is the synthetic form of a serine protease which catalyzes the conversion of plasminogen in plasmin, hence promoting clot fibrin dissolution. On this basis, researchers looked for changes in thrombus histology in AIS patients receiving intravenous thrombolysis before mechanical thrombectomy. Although some articles reported increased content in fibrin and lower content in RBCs in clots removed by stent retrieving after rtPA infusion[12,65,66], other studies did not show significant differences in terms of clot composition linked to intravenous thrombolysis[59,67,68]. Moreover, some researchers found changes in thrombus architecture or size following thrombolytic treatment. In particular, one group described the transformation of normally solid fibrin into a loose web-like state and named this phenomenon “thinning”. Moreover, clots were classified into 4 stages of lysis (0-3) depending on the extent of the observed “thinning”, and the highest stage of thinning (i.e., Stage 3) was associated with bridging therapy[69]. As to clot size, rtPA seems to reduce its cross-sectional area[65,70]. By using a different classification of fibrin age[32], changes in fibrin were also reported by Khismatullin et al.[14], with the prevalence of “old fibrin” in patients who received IVT.
The literature reports that not all arterial occlusions are responsive to rtPA, suggesting that some kind of thrombi might be resistant to IVT[71-73]. Both clinical and in vitro studies suggested that the highest fibrin and WBC content make clots less prone to rtPA action[53,66]. In particular, an outer shell composed of fibrin, platelets, VWF, and NETs would provide a protective coat against thrombolysis[74]. In thrombotic material from cerebral arteries, the presence of VWF correlates with the presence of fibrin, platelets, and neutrophils, probably enhancing their adhesion during thrombus formation[75].
IMAGING FEATURES
Given the interest of scientific community on the histopathologic composition of thrombi and their relationship with procedural outcome in AIS, the possibility to predict clot characteristics from pre-interventional imaging is an utmost relevant issue.
Non-contrast computed tomography and computed tomography angiography
Non-contrast computed tomography (NCCT) is the most widely used baseline imaging for AIS in emergency departments, due to short times of acquisition and widespread availability. Computed tomography angiography (CTA) of supra-aortic and intracranial vessels is a sensitive and specific tool to detect LVO, and it is now a standard procedure in the diagnostic workflow of AIS. We found 12 articles reporting an association between clot characteristic on CT and histological composition both on retrieved thrombi and on clot analogs[8,16,42,76-84].
Hyperdense middle cerebral artery sign (HMCAS) is a well-known sign of LVO in AIS on baseline NCCT. Its presence was proved to be related with higher content of RBCs in thrombi[8,42,76,85], while its absence is related to platelet-rich clots[77,78].
Investigators have been looking for more precise methods of identifying clot composition on pre-interventional imaging. Most of studies relating NCCT and thrombus histology showed that thrombus attenuation, measured on NCCT in Hounsfield units, increases with RBC content and is lower in fibrin-rich clots[8,16,79,84]. Furthermore, a study with clot analogs found that RBCs and, to a lesser extent, iron content are independent predictors of thrombus attenuation and suggested that maximum attenuation from axial NCCT seems to be a better marker of RBC content than the widely used mean attenuation value[83]. Future perspectives should include the use of automated technologies for the classification of occluding material, to help interventionalists choose the best technique for their patients. A machine learning system able to predict thrombus composition was developed by Hanning et al.[80] who showed that a computer-based classifier, using quantitative markers from admission imaging, can distinguish fibrin-rich and RBC-rich clots with good rates of sensitivity and specificity.
Despite occluding large vessels, thrombi can allow varying degrees of flow passage through their structure, a property defined as thrombus permeability (or perviousness). The radiological marker of thrombus perviousness results in contrast uptake by the occluding material on CTA, and its degree can be evaluated through the increase of thrombus attenuation compared with NCCT images. Some authors demonstrated that permeable thrombi are more susceptible to rtPA lysis[86] and are related to better recanalization and clinical outcome[87]. Histology of permeable thrombi is still under debate. In one study on clot analogs[84] and in a clinical study[81], fibrin-rich thrombi showed a higher increase of attenuation after contrast uptake compared to RBC-rich ones, as for a higher affinity of fibrin to contrast mean. Conversely, a larger cohort study showed that pervious clots had higher RBC and lower fibrin and WBC density[82], a finding which would better explain the higher recanalization rates reported for pervious thrombi.
Magnetic resonance imaging
Although CT is the most widely used neuroimaging in emergency setting, magnetic resonance imaging (MRI) may be essential, especially after the extension of the therapeutic time windows for AIS[88,89]. Similar to HMCAS in CT, the so-called susceptibility vessel sign (SVS), defined as a hypointense signal inside the occluded artery with a blooming artifact on MR SWI sequences, such as gradient echo (GE) sequence, can detect the occluding clots in AIS patients[90]. From a histological point of view, clots showing SVS contain a higher proportion of RBCs, while absence of this radiological sign denotes higher fibrin content[17,76]. One in vitro study on ovine clot analogs created a model to predict clot RBC content on MRI: the variation of signal intensity ratio showed an inverse correlation with RBC proportion. The statistical model to explore a predictive value was applied on fluid attenuated inversion recovery sequence, because of signal saturation on GE and SWI[91]. The higher RBC content of thrombi showing higher attenuation on CT or SVS on MRI probably reflects the hemoglobin concentration in the occluding material.
Another in vitro study on frozen cross-sections of the retrieved thrombi proposed spectroscopy techniques to detect not only RBC and fibrin composition of thrombi, but also fibrin structure and lipid content[92]. These preliminary results need confirmation through clinical studies on retrieved clots.
Other neuroimaging
The mechanical properties of clots depend on their histological composition and may influence the success of endovascular procedures[43]. Elastography imaging on optical coherence tomography and ultrasound are non-invasive techniques able to define the mechanical properties of biological tissues[93,94]. One in vitro study investigated whether acoustic radiation force optical coherence elastography (ARF-OCE) could detect thrombus histology and compared this technique with ultrasound shear wave elastography (SWE). This study demonstrated that both techniques were able to characterize clot composition and that ARF-OCE could better differentiate RBC content compared to SWE[95]. Further investigations are required to confirm whether elastography might help to choose the more suitable endovascular technique in clinical practice.
OTHER CLINICAL VARIABLES
Most of the studies on thrombus composition included AIS from occlusion of the anterior cerebral circulation, while very few data on basilar artery occlusions (BAO) are available. One study which compared 59 clots from BAO to 122 from anterior circulation found that BAO clots were significantly richer in RBCs[96]. Higher content of RBCs in BAO clots might be related to a higher incidence of large artery atherothrombosis in these patients[7-12]. However, a higher content of RBCs in thrombi from BAO was found irrespective of stroke subtype, leading the authors to conclude that different flow conditions in BAO may contribute to the evolution of thrombus, through a higher amount of fresh apposition of RBCs than in anterior circulation[96].
Regarding anterior cerebral circulation, thrombi from ICA occlusion seems to be larger than the ones retrieved from MCA with a cut off of 3 mm[97].
Thrombus formation is a dynamic and multifactorial process[98], which could theoretically also be influenced by the underlying cardiovascular risk factors. Clots from diabetic patients contained a higher percentage of fibrin and a lower percentage of RBCs, irrespective of baseline serum glucose levels, while no difference was found in VWF and WBC contents[99]. This result was confirmed in the different AIS subtypes. Diabetic patients have less efficient mechanisms of intrinsic fibrinolysis and higher resistance to rtPA[100], which could explain the prevalent fibrin composition found in thrombi retrieved from this population.
One study reported a correlation between NETs content in thrombus and worse clinical outcome at univariate analysis, which was not confirmed at multivariable analysis, probably due to the influence of other variables such as recanalization rates and etiology[25].
Worse 90-day functional outcome was reported to be associated to larger clot area[33], but this result came from a small cohort of patients with a large portion of Tandem occlusions, which are typically bound to more severe strokes. Sallustio et al.[33] analyzed fibrin structure of clots and outlined a higher prevalence of layered thrombi in more severe stroke; nevertheless, patients with the same type of thrombi had better early clinical outcome and improvement after 24 h. On the contrary, Schuhmann et al.[21] found greater VWF content in clots from patients with higher NIHSS, without any association with clinical outcome. However, the correlation between VWF and stroke severity was not confirmed by Douglas et al.[44].
Finally, one study on the morphological analysis of thrombus components focused on clot contraction[14]. Signs of intravital clot contraction, namely higher content of deformed RBCs defined as polyhedral RBCs, and an increase in platelet portion and fibrin bundles were evident in patients with moderate and severe strokes compared with the less severe (NIHSS ≤ 10) group[14]. Conversely, thrombi from less severe cases contain a newly described morphological fibrin structure called fibrin sponge, characterized by a porous amorphous structure. Moreover, Khismatullin et al.[14] found that worse long-term outcome, measured by 90-day modified Rankin Scale (mRS), directly correlated with the presence in thrombi of polyhedral RBCs and of clustered leukocytes. This last result might be consistent with presence of NETs in patients with worse clinical outcome[25]. An organized structure of the thrombus with alternating layers of RBCs and fibrin mixed with platelets was common in the group of patients with good clinical outcome (mRS 0-2)[14].
CONCLUSION
AIS is a complex and multifactorial disease consequent to different pathophysiological mechanisms. Analysis of thrombi retrieved after MT might give some hints about this complexity, but data from literature are still too heterogeneous. Nonetheless, a potential value of clot histology as a tool to both understand AIS pathogenesis and personalize reperfusion therapies would be suggested by literature [Table 1].
Summary of main finding on thrombus analysis in acute ischemic stroke according to: (A) etiology; (B) procedural variables; (C) imaging features; and (D) other clinical variables
| (A) Stroke etiological subtype | |
| Type of histological analysis on thrombi | Main finding in thrombus |
| RBC, Fibrin, and platelets content | CE: ↓ RBC content or ↑ FP content[7-14] |
| CE and CS share similar thrombus composition[7,9,10] | |
| LAA: ↑ fibrin content[16,17] | |
| Thrombin activity | CE: decreasing thrombin activity LAA: steady thrombin activity[19] |
| Inflammatory cells and markers | CE: ↑ WBC content[9,22,23] |
| LAA: ↑ CD3+ cells[24] | |
| LAA: ↑ monocyte content CE: ↑ eosinophil content CS: ↑ T and B cells[25] | |
| CE: ↑ content of NETs[25] | |
| CE: ↑ content of DNA (marker of WBC and NETs)[29] | |
| Macroscopic aspect and structure of thrombus | LAA: larger clot area[13] |
| LAA: fibrillar fibrin CE: fibrin bundles[14] | |
| (B) Thrombus histology and endovascular procedure | |
| Procedural feature | Main finding in thrombus |
| Recanalization | Successful recanalization: ↑ RBC content TICI 0-2a: ↑ Platelet content[16,42-44] |
| Shorter procedures and lower number of passages: ↑ RBC content Longer procedure and higher number of passages: ↑ FP content[8,48,49,52] | |
| Longer procedure and higher number of passages: ↑ NETs content[53] | |
| Procedural complications | Thrombus migration: ↑ RBC content and longer thrombi[55-57] |
| Secondary embolism: ↑ fibrin and ↓ RBC content[8] | |
| Secondary embolism: ↑ RBC content[48,58,59] | |
| Secondary embolism: ↑ neutrophil content[59] | |
| Intravenous thrombolysis | Bridging therapy: ↑ fibrin and ↓ RBC content[12,65,66] |
| Bridging therapy: no differences in thrombus composition[59,67,68] | |
| Bridging therapy: differences in fibrin pattern[14] | |
| Bridging therapy: reduced area[65,70] | |
| rtPA resistance: ↑ fibrin, WBC, NETs and VWF content[53,66,74] | |
| (C) Imaging features | |
| Neuroimaging technique | Main finding in thrombus |
| Non contrast CT and CT angiography | HMCAS: ↑ RBC and ↓ fibrin content[8,42,76-78,85] |
| Thrombus attenuation: directly correlated with RBC content and inversely correlated with fibrin content[8,16,79,83,84] | |
| Thrombus permeability: ↑ fibrin content[81,84] | |
| Thrombus permeability: ↑ RBC and ↓ fibrin and WBC content[82] | |
| MRI | Susceptibility vessel sign: ↑ RBC and ↓ fibrin content[17,76] |
| (D) Other clinical variables | |
| Clinical feature | Main finding on thrombus |
| Site of occlusion | Basilar artery occlusion: ↑ RBC content[96] |
| Internal carotid artery occlusion: larger thrombi compared to middle cerebral artery occlusion[97] | |
| Stroke severity and clinical outcome | Worse 90 days functional outcome: larger clot area[33] |
| Worse 90 days functional outcome: ↑ polyhedral RBC content and clustered WBC[14] | |
| Admission NIHSS > 10: ↑ polyhedral RBC, platelets, and fibrin bundles content Admission NIHSS ≤ 10: fibrin sponge[14] | |
| Admission NIHSS > 10: layered thrombus[33] |
Future research should first standardize histological methodology, which should include RBC, PLT, fibrin, WBC, VWF, and NET analyses, and then correlate histological data with imaging findings, EVT success and complication, etiology, and comorbidities. Moreover, differences in the analyzed populations should always be considered, in particular taking into account gender differences for etiology and severity of stroke[101].
In fact, some researchers have already set up large biobanks collecting thrombi, blood samples, imaging, and clinical features from AIS patients[102].
Although thrombus analysis is not yet widely used, every comprehensive stroke center performing EVT should collect occluding material and arrange internal cooperation plans with pathology and microbiology labs. This would help increase the knowledge about the possible role of thrombus histological analysis in clinical practice.
DECLARATIONS
Authors’ contributionsConceived of the presented idea: Nicolini E, Toni D
Developed the theory and performed the literature research: Nicolini E, Petraglia L, Falcou A
Analyzed and interpreted results: Nicolini E, Falcou A
Supervised the findings of this work: De Michele M, Berto I
Prepared the draft manuscript: Nicolini E
All authors discussed the results and contributed to the final manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Rohan V, Baxa J, Tupy R, et al. Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke 2014;45:2010-7.
2. Johnson CO, Nguyen M, Roth GA, et al. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:439-58.
3. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-31.
4. Meyer SF, Andersson T, Baxter B, et al; Clot Summit Group. Analyses of thrombi in acute ischemic stroke: a consensus statement on current knowledge and future directions. Int J Stroke 2017;12:606-14.
5. Kuriakose D, Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci 2020;21:7609.
6. Staessens S, Fitzgerald S, Andersson T, et al. Histological stroke clot analysis after thrombectomy: technical aspects and recommendations. Int J Stroke 2020;15:467-76.
7. Nouh A, Mehta T, Hussain M, Song X, Ollenschleger M. Clot composition of embolic strokes of undetermined source: a feasibility study. BMC Neurol 2020;20:383.
8. Sporns PB, Hanning U, Schwindt W, et al. Ischemic stroke: histological thrombus composition and pre-interventional CT attenuation are associated with intervention time and rate of secondary embolism. Cerebrovasc Dis 2017;44:344-50.
9. Boeckh-Behrens T, Kleine JF, Zimmer C, et al. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke 2016;47:1864-71.
10. Liao Y, Guan M, Liang D, et al. Differences in pathological composition among large artery occlusion cerebral thrombi, valvular heart disease atrial thrombi and carotid endarterectomy plaques. Front Neurol 2020;11:811.
11. Niesten JM, van der Schaaf IC, van Dam L, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One 2014;9:e88882.
12. Goebel J, Gaida BJ, Wanke I, et al. Is histologic thrombus composition in acute stroke linked to stroke etiology or to interventional parameters? AJNR Am J Neuroradiol 2020;41:650-7.
13. Duffy S, McCarthy R, Farrell M, et al. Per-pass analysis of thrombus composition in patients with acute ischemic stroke undergoing mechanical thrombectomy. Stroke 2019;50:1156-63.
14. Khismatullin RR, Nagaswami C, Shakirova AZ, et al. Quantitative morphology of cerebral thrombi related to intravital contraction and clinical features of ischemic stroke. Stroke 2020;51:3640-50.
15. Kim B, Kim YM, Jin SC, et al. Development of a predictive scale for cardioembolic stroke using extracted thrombi and angiographic findings. J Clin Neurosci 2020;73:224-30.
16. Fitzgerald S, Dai D, Wang S, et al. Platelet-rich emboli in cerebral large vessel occlusion are associated with a large artery atherosclerosis source. Stroke 2019;50:1907-10.
17. Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-echo MRI. AJNR Am J Neuroradiol 2015;36:1756-62.
18. Caplan LR. Antiplatelet therapy in stroke prevention: present and future. Cerebrovasc Dis 2006;21 Suppl 1:1-6.
19. Itsekson Hayosh Z, Abu Bandora E, Shelestovich N, et al. In-thrombus thrombin secretion: a new diagnostic marker of atrial fibrillation in cryptogenic stroke. J Neurointerv Surg 2020:neurintsurg-2020.
20. Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in stroke brain damage. Stroke 2016;47:1165-72.
21. Schuhmann MK, Gunreben I, Kleinschnitz C, Kraft P. Immunohistochemical analysis of cerebral thrombi retrieved by mechanical thrombectomy from patients with acute ischemic stroke. Int J Mol Sci 2016;17:298.
22. Sporns PB, Hanning U, Schwindt W, et al. Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke 2017;48:2206-10.
23. Boeckh-Behrens T, Schubert M, Förschler A, et al. The impact of histological clot composition in embolic stroke. Clin Neuroradiol 2016;26:189-97.
24. Dargazanli C, Rigau V, Eker O, et al. High CD3+ cells in intracranial thrombi represent a biomarker of atherothrombotic stroke. PLoS One 2016;11:e0154945.
25. Novotny J, Oberdieck P, Titova A, et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology 2020;94:e2346-60.
26. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532-5.
27. Laridan E, Denorme F, Desender L, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol 2017;82:223-32.
28. Savchenko AS, Martinod K, Seidman MA, et al. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost 2014;12:860-70.
29. Meglio L, Desilles JP, Solonomenjanahary M, et al; compoCLOT study group†. DNA content in ischemic stroke thrombi can help identify cardioembolic strokes among strokes of undetermined cause. Stroke 2020;51:2810-6.
30. Valente M, Saab J, Cordato D, Manning N, Cappelen-Smith C. The diagnostic utility of routine clot analysis after endovascular thrombectomy in a patient with systemic lupus erythematosus and antiphospholipid syndrome. J Clin Neurosci 2019;70:247-9.
31. Sgreccia A, Duchmann Z, Desilles JP, et al. COMPO-Clot Investigators. Association between acute ischemic stroke etiology and macroscopic aspect of retrieved clots: is a clot's color a warning light for underlying pathologies? J Neurointerv Surg 2019;11:1197-200.
32. Khismatullin RR, Shakirova AZ, Weisel JW, Litvinov RI. Age-dependent differential staining of fibrin in blood clots and thrombi. BioNanoSci 2020;10:370-4.
33. Sallustio F, Arnò N, Legge S di, et al. Histological features of intracranial thrombo - emboli predict response to endovascular therapy for acute ischemic stroke. J Neurol Disord Stroke 2015;3:1-5.
34. Bhaskar S, Saab J, Cappelen-Smith C, et al. Clot histopathology in ischemic stroke with infective endocarditis. Can J Neurol Sci 2019;46:331-6.
35. Hernández-Fernández F, Rojas-Bartolomé L, García-García J, et al. Histopathological and bacteriological analysis of thrombus material extracted during mechanical thrombectomy in acute stroke patients. Cardiovasc Intervent Radiol 2017;40:1851-60.
36. Abdel-Wahed L, Shaban A, Hayakawa M, Limaye K. Retrieved arterial clot helps guide antibiotic therapy in infective endocarditis. Am J Med 2019;132:e795-6.
37. Khashim Z, Fitzgerald S, Kadirvel R, et al. Clots retrieved by mechanical thrombectomy from acute ischemic stroke patients show no evidence of bacteria. Interv Neuroradiol 2019;25:502-7.
38. Rosenfeld ME. Inflammation and atherosclerosis: direct versus indirect mechanisms. Curr Opin Pharmacol 2013;13:154-60.
39. Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke 2001;32:2575-9.
40. Patrakka O, Pienimäki JP, Tuomisto S, et al. Oral bacterial signatures in cerebral thrombi of patients with acute ischemic stroke treated with thrombectomy. J Am Heart Assoc 2019;8:e012330.
41. Higashida RT, Furlan AJ, Roberts H, et al. Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109-37.
42. Shin JW, Jeong HS, Kwon HJ, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS One 2018;13:e0197492.
43. Hashimoto T, Hayakawa M, Funatsu N, et al. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke 2016;47:3035-7.
44. Douglas A, Fitzgerald S, Mereuta OM, et al. Platelet-rich emboli are associated with von Willebrand factor levels and have poorer revascularization outcomes. J Neurointerv Surg 2020;12:557-62.
45. Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg 2018;10:34-8.
46. Kim OV, Litvinov RI, Alber MS, Weisel JW. Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nat Commun 2017;8:1274.
47. Staessens S, Denorme F, Francois O, et al. Structural analysis of ischemic stroke thrombi: histological indications for therapy resistance. Haematologica 2020;105:498-507.
48. Weafer FM, Duffy S, Machado I, et al. Characterization of strut indentation during mechanical thrombectomy in acute ischemic stroke clot analogs. J Neurointerv Surg 2019;11:891-7.
49. Maekawa K, Shibata M, Nakajima H, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra 2018;8:39-49.
50. Fitzgerald S, Rossi R, Mereuta OM, et al. Per-pass analysis of acute ischemic stroke clots: impact of stroke etiology on extracted clot area and histological composition. J Neurointerv Surg 2020;neurintsurg-2020.
51. Johnson S, Chueh J, Gounis MJ, et al. Mechanical behavior of in vitro blood clots and the implications for acute ischemic stroke treatment. J Neurointerv Surg 2020;12:853-7.
52. Fitzgerald S, Ryan D, Thornton J, Nogueira RG. Preclinical evaluation of Millipede 088 intracranial aspiration catheter in cadaver and in vitro thrombectomy models. J Neurointerv Surg 2021;13:447-52.
53. Ducroux C, Di Meglio L, Loyau S, et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018;49:754-7.
54. Longstaff C, Varjú I, Sótonyi P, et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem 2013;288:6946-56.
55. Sporns PB, Jeibmann A, Minnerup J, et al. Histological clot composition is associated with preinterventional clot migration in acute stroke patients. Stroke 2019;50:2065-71.
56. Sporns PB, Krähling H, Psychogios MN, et al. Small thrombus size, thrombus composition, and poor collaterals predict pre-interventional thrombus migration. J Neurointerv Surg 2021;13:409-14.
57. Maegerlein C, Friedrich B, Berndt M, et al. Impact of histological thrombus composition on preinterventional thrombus migration in patients with acute occlusions of the middle cerebral artery. Interv Neuroradiol 2018;24:70-5.
58. Ye G, Qi P, Chen K, et al. Risk of secondary embolism events during mechanical thrombectomy for acute ischemic stroke: a single-center study based on histological analysis. Clin Neurol Neurosurg 2020;193:105749.
59. Kaesmacher J, Boeckh-Behrens T, Simon S, et al. Risk of thrombus fragmentation during endovascular stroke treatment. AJNR Am J Neuroradiol 2017;38:991-8.
60. Kirchhofer D, Riederer MA, Baumgartner HR. Specific accumulation of circulating monocytes and polymorphonuclear leukocytes on platelet thrombi in a vascular injury model. Blood 1997;89:1270-8.
61. Imamura T, Kaneda H, Nakamura S. New functions of neutrophils in the arthus reaction: expression of tissue factor, the clotting initiator, and fibrinolysis by elastase. Lab Invest 2002;82:1287-95.
62. Komorowicz E, Kolev K, Léránt I, Machovich R. Flow rate-modulated dissolution of fibrin with clot-embedded and circulating proteases. Circ Res 1998;82:1102-8.
63. Singh P, Doostkam S, Reinhard M, Ivanovas V, Taschner CA. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke: does stent-retriever cause intimal damage? Stroke 2013;44:1720-2.
64. Funatsu N, Hayakawa M, Hashimoto T, et al. Vascular wall components in thrombi obtained by acute stroke thrombectomy: clinical significance and related factors. J Neurointerv Surg 2019;11:232-6.
65. Horie N, Shobayashi K, Morofuji Y, et al. Impact of mechanical thrombectomy device on thrombus histology in acute embolic stroke. World Neurosurg 2019;132:e418-22.
66. Choi MH, Park GH, Lee JS, et al. Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke 2018;49:652-9.
67. Gong L, Zheng X, Feng L, et al. Bridging therapy versus direct mechanical thrombectomy in patients with acute ischemic stroke due to middle cerebral artery occlusion: a clinical - histological analysis of retrieved thrombi. Cell Transplant 2019;28:684-90.
68. Ahn SH, Hong R, Choo IS, et al. Histologic features of acute thrombi retrieved from stroke patients during mechanical reperfusion therapy. Int J Stroke 2016;11:1036-44.
69. Krajíčková D, Krajina A, Šteiner I, et al. Fibrin clot architecture in acute ischemic stroke treated with mechanical thrombectomy with stent-retrievers - cohort study. Circ J 2018;82:866-73.
70. Rossi R, Fitzgerald S, Molina S, et al. The administration of rtPA before mechanical thrombectomy in acute ischemic stroke patients is associated with a significant reduction of the retrieved clot area but it does not influence revascularization outcome. J Thromb Thrombolysis 2021;51:545-51.
71. Kim YD, Nam HS, Kim SH, et al. Time-dependent thrombus resolution after tissue-type plasminogen activator in patients with stroke and mice. Stroke 2015;46:1877-82.
72. Collet JP, Allali Y, Lesty C, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol 2006;26:2567-73.
73. Mehta BP, Nogueira RG. Should clot composition affect choice of endovascular therapy? Neurology 2012;79:S63-7.
74. Di Meglio L, Desilles JP, Ollivier V, et al. Acute ischemic stroke thrombi have an outer shell that impairs fibrinolysis. Neurology 2019;93:e1686-98.
75. Prochazka V, Jonszta T, Czerny D, et al. The role of von willebrand factor, ADAMTS13, and cerebral artery thrombus composition in patient outcome following mechanical thrombectomy for acute ischemic stroke. Med Sci Monit 2018;24:3929-45.
76. Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011;42:1237-43.
77. Fitzgerald ST, Wang S, Dai D, et al. Platelet-rich clots as identified by Martius Scarlet Blue staining are isodense on NCCT. J Neurointerv Surg 2019;11:1145-9.
78. Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg 2017;9:529-34.
79. Songsaeng D, Kaeowirun T, Sakarunchai I, et al. Efficacy of thrombus density on noninvasive computed tomography neuroimaging for predicting thrombus pathology and patient outcome after mechanical thrombectomy in acute ischemic stroke. Asian J Neurosurg 2019;14:795-800.
80. Hanning U, Sporns PB, Psychogios MN, et al. Imaging-based prediction of histological clot composition from admission CT imaging. J Neurointerv Surg 2021;neurintsurg-2020.
81. Berndt M, Friedrich B, Maegerlein C, et al. Thrombus permeability in admission computed tomographic imaging indicates stroke pathogenesis based on thrombus histology. Stroke 2018;49:2674-82.
82. Benson JC, Fitzgerald ST, Kadirvel R, et al. Clot permeability and histopathology: is a clot's perviousness on CT imaging correlated with its histologic composition? J Neurointerv Surg 2020;12:38-42.
83. Velasco Gonzalez A, Buerke B, Görlich D, et al. Clot analog attenuation in non-contrast CT predicts histology: an experimental study using machine learning. Transl Stroke Res 2020;11:940-9.
84. Borggrefe J, Kottlors J, Mirza M, et al. Differentiation of clot composition using conventional and dual-energy computed tomography. Clin Neuroradiol 2018;28:515-22.
85. Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol 2015;42:86-92.
86. Santos EM, Dankbaar JW, Treurniet KM, et al. DUST Investigators. Permeable thrombi are associated with higher intravenous recombinant tissue-type plasminogen activator treatment success in patients with acute ischemic stroke. Stroke 2016;47:2058-65.
87. Santos EM, Marquering HA, den Blanken MD, et al. MR CLEAN Investigators. Thrombus permeability is associated with improved functional outcome and recanalization in patients with ischemic stroke. Stroke 2016;47:732-41.
88. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021;6:I-LXII.
89. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic strokeendorsed by stroke alliance for Europe (SAFE). Eur Stroke J 2019;4:6-12.
90. Rovira A, Orellana P, Alvarez-Sabín J, et al. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology 2004;232:466-73.
91. Janot K, Oliveira TR, Fromont-Hankard G, et al. Quantitative estimation of thrombus-erythrocytes using MRI. A phantom study with clot analogs and analysis by statistic regression models. J Neurointerv Surg 2020;12:181-5.
92. Blat A, Dybas J, Chrabaszcz K, et al. FTIR, Raman and AFM characterization of the clinically valid biochemical parameters of the thrombi in acute ischemic stroke. Sci Rep 2019;9:15475.
93. Li C, Guan G, Reif R, Huang Z, Wang RK. Determining elastic properties of skin by measuring surface waves from an impulse mechanical stimulus using phase-sensitive optical coherence tomography. J R Soc Interface 2012;9:831-41.
94. Liu X, Li N, Wen C. Effect of pathological heterogeneity on shear wave elasticity imaging in the staging of deep venous thrombosis. PLoS One 2017;12:e0179103.
95. Liu HC, Abbasi M, Ding YH, et al. Characterizing blood clots using acoustic radiation force optical coherence elastography and ultrasound shear wave elastography. Phys Med Biol 2021;66:035013.
96. Berndt M, Poppert H, Steiger K, et al. Thrombus histology of basilar artery occlusions: are there differences to the anterior circulation? Clin Neuroradiol 2020; doi: 10.1007/s00062-020-00964-5.
97. Marder VJ, Chute DJ, Starkman S, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006;37:2086-93.
98. Gersh KC, Zaitsev S, Muzykantov V, Cines DB, Weisel JW. The spatial dynamics of fibrin clot dissolution catalyzed by erythrocyte-bound vs. free fibrinolytics. J Thromb Haemost 2010;8:1066-74.
99. Ye G, Gao Q, Qi P, et al. The role of diabetes mellitus on the thrombus composition in patients with acute ischemic stroke. Interv Neuroradiol 2020;26:329-36.
101. Arboix A, Cartanyà A, Lowak M, et al. Gender differences and woman-specific trends in acute stroke: results from a hospital-based registry (1986-2009). Clin Neurol Neurosurg 2014;127:19-24.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Nicolini E, De Michele M, Falcou A, Petraglia L, Berto I, Toni D. Histological analysis of thrombi retrieved after acute ischemic stroke from large vessel occlusion: from research to clinical practice. Vessel Plus 2022;6:2. http://dx.doi.org/10.20517/2574-1209.2021.78
AMA Style
Nicolini E, De Michele M, Falcou A, Petraglia L, Berto I, Toni D. Histological analysis of thrombi retrieved after acute ischemic stroke from large vessel occlusion: from research to clinical practice. Vessel Plus. 2022; 6: 2. http://dx.doi.org/10.20517/2574-1209.2021.78
Chicago/Turabian Style
Nicolini, Ettore, Manuela De Michele, Anne Falcou, Luca Petraglia, Irene Berto, Danilo Toni. 2022. "Histological analysis of thrombi retrieved after acute ischemic stroke from large vessel occlusion: from research to clinical practice" Vessel Plus. 6: 2. http://dx.doi.org/10.20517/2574-1209.2021.78
ACS Style
Nicolini, E.; De Michele M.; Falcou A.; Petraglia L.; Berto I.; Toni D. Histological analysis of thrombi retrieved after acute ischemic stroke from large vessel occlusion: from research to clinical practice. Vessel Plus. 2022, 6, 2. http://dx.doi.org/10.20517/2574-1209.2021.78
About This Article
Copyright
Data & Comments
Data
 Cite This Article 12 clicks
Cite This Article 12 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.