Nuclear medicine techniques for the diagnosis of cardiac amyloidosis: the state of the art
Abstract
Amyloidosis is a disease characterized by the deposition of amorphous protein material in the extracellular space which leads to progressive dysfunction of the affected organ. The forms of amyloidosis that most frequently involve the heart are transthyretin amyloidosis (ATTR) and immunoglobulin light chain amyloidosis (AL). Nuclear medicine offers numerous imaging techniques for the evaluation of patients with cardiac amyloidosis, and in the last decade osteophilic tracer scintigraphy has assumed a fundamental role in the diagnostic process of this disease. New PET radiopharmaceuticals for the detection of amyloid deposits are proving very effective in diagnosing the presence of AL amyloidosis and could soon allow a differential diagnosis without the need for invasive and potentially risky techniques such as endomyocardial biopsy.
Keywords
INTRODUCTION
Amyloidosis is an infiltrative and restrictive disease due to interstitial deposition of misfolded proteins which leads to organ damage and functional impairment. In most patients with cardiac amyloidosis, the diagnosis is made only in advanced clinical stages when ventricular function is so compromised that most of the therapeutic strategies are useless; a correct and prompt clinical diagnosis remains the only possibility to improve the outcome of this pathology, which is not as rare as was believed in the past. Nuclear medicine allows, in a non-invasive way, obtaining information on ventricular perfusion, function, and innervation; myocardial metabolism; and the presence of amyloid deposits. The diagnosis of cardiac amyloidosis requires the evaluation of clinical and bio-humoral parameters a series of instrumental evaluations, and often a bioptic confirmation, sometimes by endomyocardial biopsy.
For the diagnosis of transthyretin cardiac amyloidosis (ATTR), it is now possible to perform a scintigraphic evaluation using bone-seeking radiopharmaceuticals labeled with metastable technetium 99 (99mTc-PYP, 99mTc-DPD, and 99mTc-HMDP); this approach, when a monoclonal disease is excluded, allows a definitive diagnosis without the need for a histological examination.
When the scintigraphy with osteotropic radiopharmaceuticals is negative or in the presence of a monoclonal disease, the definitive diagnosis of cardiac amyloidosis cannot be reached without performing further instrumental investigations and eventually with a bioptic confirmation.
In the last 10 years, the development of new PET radiopharmaceuticals for the detection of amyloid deposits has opened a new path for the differential diagnosis of cardiac amyloidosis.
VENTRICULAR PERFUSION
Patients with cardiac amyloidosis (CA) can have anginal symptoms in the absence of coronary artery disease, as amyloid deposition leads to alterations of the endothelial function and microvascular dysfunction before the onset of pseudo-hypertrophy. Cardiac scintigraphy with Thallium-201 (201Tl) and 99mTc-labeled radiopharmaceuticals (Sestamibi and Tetrofosmin) is a very accurate diagnostic tool of wide clinical use for the evaluation of myocardial perfusion and viability, but its use in CA has been limited to a few observations[1,2]. Myocardial bone-seeking radiotracer uptake has been observed in a patient without any scintigraphic evidence of myocardial ischemia and with impaired ejection-fraction[3], suggesting that combined imaging may be useful in assessing the extent of organ damage. Kodama et al.[4] evaluated 5 patients with CA and 12 control subjects by 201Tl cardiac scintigraphy, demonstrating that myocardial regions affected by amyloid deposition are characterized by a mixture of viable and necrotic tissue; 210Tl washout was significantly increased in CA patients compared to control subjects, particularly in 4 patients who died within a year. The less evidence for the use of perfusion scintigraphy in the evaluation of patients with CA explains its limited clinical use in this kind of patients.
VENTRICULAR FUNCTION
The first nuclear image of CA was obtained in 1968 by Wolfgang Hauser; the author performed a radio-isotopic ventriculography [blood pool gating (BPG)] with 99mTc-labeled serum albumin in a patient with autoptic diagnosis of CA. BPG was used for the identification of pericardial effusion, and the researcher noticed that the patient showed an increased inter-ventricular septum thickness which was described as a scintigraphic sign of infiltrative heart disease[5]. BPG can provide other parameters such as times and rates of ventricular filling and emptying; these parameters have been used in the differential diagnostics between cardiac and pericardial restrictive pathologies. Gerson et al.[6] observed that patients with pericardial constriction showed higher peak filling rate values when compared to patients with restrictive heart disease. Hongo et al.[7] used BPG to evaluate left ventricular diastolic function in 17 patients with familial polyneuropathy, demonstrating a lower peak filling rate associated with a significant delay in ventricular filling when compared to a control group. More recently, Clements et al.[8] evaluated the differences in ventricular filling rate among three groups of patients with pulmonary disease, cardiac amyloidosis, and pericarditis and a control group, concluding that variations in ventricular filling rate can be used for differential diagnosis. Nowadays, gated-SPECT with 99mTc-Tetrofosmin or 99mTc-Sestamibi is a widely used technique to obtain perfusion and function parameters, and it also allows obtaining data on diastolic function, which is often compromised earlier than systolic function in patients with CA. However, radiation-free morphological imaging, such as cardiac echocardiography and magnetic resonance, is to be preferred in defining ventricular function of patient with known or suspected AC.
METABOLISM
The study of cellular metabolism with conventional nuclear medicine techniques using radiopharmaceuticals such as 123I-β-methyl-iodophenyl-pentadecanoic acid (123I-BMIPP) and 99mTc-pentavalent dimercaptosuccinic acid (99mTc-V-DMSA) has also been evaluated for the diagnosis of CA[9,10]. Despite the promising results of these methods, the use of these radiopharmaceuticals in clinical practice has not become common due to their limited availability and the non-optimal quality of the images obtained.
In nuclear medicine, the predominant role in the study of cellular metabolism is certainly played by PET with 18F-FluoroDeoxyGlucose (FDG); this radiopharmaceutical is widely used for PET studies in oncology, as the degree of tissue uptake of the radiopharmaceutical is directly related to the cellular glycolytic activity and, consequently, the degree of disease activity and its degree of de-differentiation.
Despite the widespread availability of PET/CT scanners and FDG, there is little evidence in the literature on the usefulness of the study of glucose metabolism in CA, which is limited to case reports[11,12].
Since the vital myocardium physiologically metabolizes glucose as an energy substrate, PET studies for the evaluation of CA should require a specific preparation by means of a low-carbohydrate and high-fat diet in the 12 h preceding the examination to obtain a shift of the myocardial metabolism towards fatty acids: in this way, areas of FDG uptake could be referred to pathologic processes related to the presence of amyloid similarly to what is described for cardiac sarcoidosis[13].
In any case, the presence of FDG uptake areas would not be a direct sign of the presence of amyloid substance but possibly an indirect sign linked to the presence of inflammation; due to its reduced specificity, this technique has not found wide use in the diagnostic process of CA.
MYOCARDIAL INNERVATION
Patients with amyloidosis often develop degeneration of cardiac autonomic innervation, which leads to cardiac rhythm alterations; this occurrence is frequent in patients with ATTR, particularly in hereditary forms, and with AL.
123I-metaiodobenzylguanidine (123I-MIBG) is a chemically modified analog of noradrenaline which is stored within the presynaptic vesicles of the sympathetic terminations which allows a non-invasive assessment of myocardial innervation. Tanaka et al.[14] demonstrated that, in patients with a familial form of amyloidosis, myocardial sympathetic denervation could occur even before the development of pseudohypertrophy or left ventricular dysfunction. Patients suffering from AL amyloidosis with cardiac involvement also have a reduction in cardiac uptake, but it is less than that of healthy controls or those of transthyretin forms[15-17]; however, since the degree of denervation presents an extensive overlap between AL and ATTR forms, it is not possible to use 123-MIBG scintigraphy to formulate a differential diagnosis between the two types of CA[17]. However, the presence of impaired cardiac sympathetic innervation is a worsening prognostic factor and 123I-MIBG scintigraphy can provide important information on patient’s outcome; in fact, the reduction of the heart/mediastinal uptake ratio has been shown to be an independent prognostic indicator with a 42% increased mortality in patients with H/R < 1.6[18]. Despite these interesting results, 123I-MIBG scintigraphy is rarely used in the diagnostic work-up of CA.
RADIOPHARMACEUTICALS FOR AMYLOID SUBSTANCE
All amyloid deposits contain a non-fibrillar glycoprotein called the “P component of the amyloid”. The P component of serum amyloid labeled with 123I (123I-SAP) is a scintigraphic radiopharmaceutical which binds to all types of amyloidogenic fibrils and can be used to identify the presence and distribution of amyloid deposits[19,20]. Hazenberg et al.[20] evaluated the diagnostic accuracy of this method: the sensitivity was 90%, 90%, and 48% in subjects with biopsy evidence of AA, AL, and ATTR amyloidosis, respectively, with a specificity of 93%. Nevertheless, cardiac involvement was not identified in any patient. For this reason, in addition to the poor quality of the images obtained, the cost of the radiopharmaceutical, and its limited availability in many countries, 123I-SAP is not widely used for the assessment of CA.
A radiopharmaceutical that is more available, more easily labeled, and with a more favorable energy peak and has been used in the past is 99mTc-aprotinin. Aprotinin is a protease inhibitor which accumulates in the tissues affected by amyloid substance deposition and can allow visualizing CA[21]. Unfortunately, even this radiopharmaceutical has not been shown to have a high binding specificity for amyloid deposits in the heart; in fact, in patients with biopsy-proven CA, the identification rate of cardiac involvement was between 36% and 44%[21,22].
Positron-emitting radiopharmaceuticals with binding affinity for amyloid all originate as in vivo tracers of cerebral beta-amyloid deposits for diagnostic confirmation of Alzheimer’s disease; some of these radiopharmaceuticals have subsequently also shown binding affinity for myocardial amyloid, both in the context of a systemic amyloidosis with cardiac involvement and in the case of isolated CA.
The first PET radiopharmaceutical for the study of amyloid deposits was patented in the early 2000s under the name of Pittsburgh Compound B (PiB); this radiopharmaceutical is a derivative of Thioflavine T (a dye for histological preparations that binds specifically to amyloid deposits) and is labeled with 11C[23]. The first prospective study performed on a small group of subjects to evaluate the usefulness of PiB in the diagnosis of CA was published in 2013[24]. In this study, the authors described a significant early myocardial uptake of the radiopharmaceutical (approximately 15-25 min after injection) in all 10 patients with amyloidosis and in no control subjects (n = 5), suggesting a possible use of this method for the diagnosis of cardiac involvement in the case of systemic amyloidosis. Unfortunately, the short decay time of 11C allows the use of this radiopharmaceutical only in centers equipped with a cyclotron.
Since 2009, some PET radiopharmaceuticals labeled with 18F with high affinity for beta-amyloid have been developed. The first of these new radiopharmaceuticals is 18F-Florbetapir, a structural analog of stilbenic dyes used in histology[25]; as for PiB, this radiopharmaceutical has also been developed for the in vivo diagnosis of AD and studies performed in humans have demonstrated its usefulness in this sense, making it the first available 18F-labeled PET tracer for brain amyloid[26,27]. The first pilot study demonstrating the affinity of 18F-Florbetapir for myocardial amyloid deposits was published in 2014[28]; the authors evaluated 14 subjects including 9 patients with cardiac amyloidosis and 5 control patients with non-infiltrative hypertrophic cardiomyopathy. Patients with cardiac amyloidosis showed significantly greater myocardial uptake of the radiopharmaceutical than controls both in SUV values and in target-to-background ratios; image analysis did not allow a differential diagnosis between AL and ATTR, but the higher myocardial retention index in the group of patients with AL suggested a higher avidity of AL than ATTR for Florbetapir.
Another 18F-labeled PET radiopharmaceutical for in vivo detection of amyloid deposits is 18F-Flutemetamol. This radiopharmaceutical is a structural analog of PiB offering much wider accessibility for both clinical and research use due to its longer physical half-life[29]. The first observation of myocardial uptake of 18F-Flutemetamol in CA was published in 2014 and referred to a single case of AL compared with 2 healthy volunteers[30]; a pilot study performed in 2019 on nine patients with amyloidosis compared with 3 control subjects described a significantly greater myocardial accumulation of the radiopharmaceutical in subjects with cardiac amyloidosis compared to controls[31]. More recently, Möckelind et al.[32] evaluated the use of 18F-Flutemetamol in a group of 21 selected patients affected by a hereditary form of ATTR with negative osteophilic tracer scintigraphy compared with 6 control subjects. Patients with ATTR showed high myocardial radiopharmaceutical uptake, and the method was able to identify patients with high accuracy (sensitivity, 88%; specificity, 100%). Möckelind et al.[32] concluded that this method could be useful for the evaluation of patients with clinical suspicion of ATTR and negative 99mTc-DPD scintigraphy.
The last radiopharmaceutical labeled with 18F for in vivo detection of amyloid deposits is 18F-Florbetaben[33]. This radiopharmaceutical has also been shown to have affinity for cardiac amyloid deposits, and, in 2016, the first study in this area was published[34]. In this pilot study, the authors compared a heterogeneous group of patients with CA (5 AL and 5 ATTR) with a control group consisting of patients with hypertensive heart disease; they concluded that this radiopharmaceutical may represent a promising tool for the diagnosis of cardiac amyloidosis, without any evidence of its ability to differentiate between AL and ATTR.
More recently, Kircher et al.[35] evaluated the diagnostic performance of 18F-Florbetaben in a group of 14 patients with CA (5 ATTR, 8 AL, and 1 AA) and 8 patients with cardiomyopathy. In 4 patients, they were also able to evaluate the PET/CT data during the follow-up. The results indicate that PET/CT with 18F-Florbetaben can discriminate between CA and non-infiltrative cardiomyopathy and that the myocardial retention index of the radiopharmaceutical is higher in AL than ATTR. Furthermore, the results derived from the evaluation of the small group of patients with re-evaluation after therapy seem to suggest a potential role of this radiopharmaceutical for the evaluation of the response to therapy[35].
All studies described thus far have taken into consideration data obtained in very early stages of the tissue distribution of the radiopharmaceutical.
A recently published work evaluates the diagnostic performance of 18F-Florbetaben PET/CT in a group of 40 patients with CA (20 AL and 20 ATTR) compared with a control group of 20 patients with non-infiltrative hypertrophic cardiomyopathy[36]. The authors evaluated quantitative parameters derived from dynamic acquisitions lasting 60 min and four static reconstructions lasting 10 min obtained, respectively, 5, 30, 50, and 110 min after the injection of the radiopharmaceutical. All quantitative parameters were significantly greater in patients with AL than in those with ATTR and controls. Moreover, the washout rate of the radiopharmaceutical between 5 and 60 min after injection was significantly higher in the ATTR group and the controls compared to the AL group, suggesting a greater affinity of the radiopharmaceutical for the amyloid substance derived from immunoglobulin light chains. The authors concluded that a late evaluation of the myocardial uptake of this radiopharmaceutical obtained at least 30 min after injection allows identifying the AL forms, differentiating them from ATTR and mimicking conditions.
OSTEOPHILIC RADIOPHARMACEUTICALS
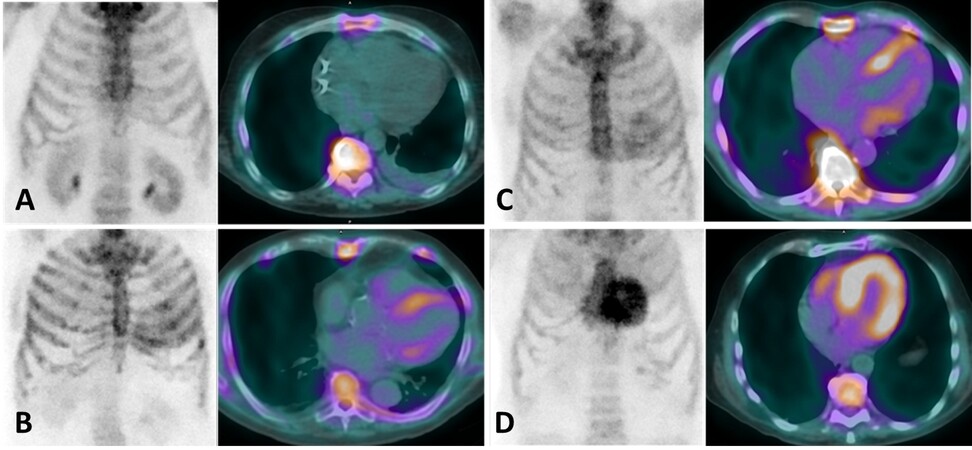
Some radiopharmaceuticals originally developed to study bone metabolism and labeled with 99mTc have been successfully used for the demonstration of amyloid cardiac involvement. These radiopharmaceuticals are 99mTc-3,3-diphosphono-1,2propanodicarboxylic acid (99mTc-DPD), 99mTc-hydroxymethylene diphosphonate (99mTc-HMDP), and 99mTc-pyrophosphate (99mTc-PYP). The first observation of 99mTc-DPD cardiac uptake in patients with AC was reported in 1977 by Kula et al.[37]. In the following decades, the number of observations supporting the usefulness of these radiopharmaceuticals for the diagnosis of CA has progressively increased. In 2005, Perugini et al.[38] demonstrated the high accuracy of cardiac retention of 99mTc-DPD in identifying patients with ATTR: cardiac uptake of the radiopharmaceutical was absent in controls and patients with AL, therefore 99mTc-DPD scintigraphy identified 100% of ATTR. In the same paper, a score of myocardial uptakes known as the “Perugini score” was proposed; this score is now widely used in clinical practice and provides a four-point scale, from 0 to 3, as described in Figure 1. In a later study performed in a larger population, Rapezzi et al.[39] semi-quantitatively evaluated myocardial uptake of 99mTc-DPD [heart/total body retention ratio (H/WB)]; with this approach, the accuracy resulted lower than previously reported as one third of patients with AL amyloidosis showed mild cardiac uptake of the radiopharmaceutical. By using a semiquantitative cut-off of moderate to high uptake (Scores 2-3), 99mTc-DPD scintigraphy showed 100% and 88% sensitivity and specificity, respectively. Cardiac retention of 99mTc-DPD has been shown to correlate with inter-ventricular septum thickening and the severity of the contractile function impairment[40]; moreover, a marked cardiac retention of the radiopharmaceutical has been associated with a high risk of future cardiac events and poor prognosis[41].
Figure 1. Scintigraphic visual score of the radiopharmaceutical uptake as originally proposed by Perugini et al.[38]: (A) Score 0, no cardiac uptake; (B) Score 1, mild cardiac uptake lower than bone; (C) Score 2, moderate cardiac uptake equal to bone; and (D) Score 3, intense cardiac uptake higher than bone.
99mTc-PYP is another commonly used radiopharmaceutical for bone imaging which has been shown to be useful for diagnosing CA. In 1983, Falk et al.[42] evaluated 20 patients with CA and 10 control subjects using 99mTc-PYP, reporting a high cardiac uptake of the radiopharmaceutical in 9 of 11 patients with echocardiograms suggestive of amyloidosis and in only 2 patients without pseudohypertrophy. None of the 10 control subjects showed cardiac pyrophosphate uptake, suggesting that 99mTc-PYP scintigraphy could represent a non-invasive imaging of CA[42]. In a study on a larger sample of patients with a biopsy-proven CA (12 AL, 16 ATTR wild-type, and 17 ATTR familial), both the evaluation through a visual score and a semiquantitative measure of myocardial uptake (heart/contralateral chest activity ratio) correlated with ventricular wall thicknesses and myocardial mass and were higher in subjects with ATTR. The sensitivity and specificity of the method were 97% and 100%, respectively[43].
Recently, Cappelli et al.[44] evaluated the diagnostic performance of 99mTc-HMDP in a population of 65 patients with histological diagnosis of cardiac amyloidosis (39 ATTR and 26 AL) and in 20 subjects with non-amyloidotic ventricular hypertrophy. The visual score imaging evaluation provided a diagnostic sensitivity of 100% and a specificity of 96%. None of the subjects with non-amyloidotic left ventricular hypertrophy had significant uptake of the radiopharmaceutical. Using a semiquantitative approach (heart/total body activity ratio) and a threshold of > 3.3, a diagnostic accuracy of 100% was obtained in the diagnosis of ATTR. The authors concluded that 99mTc-HMDP scintigraphy can be used in the differential diagnosis of AC with levels of accuracy comparable to those of other osteophilic radiopharmaceuticals previously evaluated.
The real milestone study for this method was conducted by Gillmore et al.[45], who analyzed the scintigraphic results obtained with the three available 99mTc-labeled radiopharmaceuticals in more than 1000 patients with suspected AC. Bone scan had a diagnostic sensitivity greater than 99% and a specificity of 86% in identifying cardiac involvement from ATTR, with false positives mostly due to mild uptake patterns in patients with AL amyloidosis. When considering moderate to high scintigraphic uptake patterns with the absence of serum and urinary monoclonal protein, a diagnostic specificity and a positive predictive value for ATTR of 100% were obtained. Therefore, the authors suggested that a positive scintigraphic imaging in patients with symptoms of heart failure with preserved ejection fraction, myocardial pseudohypertrophy, and absence of serum and urinary monoclonal component may allow the diagnosis of ATTR without the need for confirmation by biopsy. These results have recently led to a clinical consensus which reserves particular importance to scintigraphy in the non-invasive diagnostic pathway of CA[46]. When plasma cell dyscrasia is present and bone scan is positive, histological diagnosis remains necessary since up to 20% of patients with AL may have significant cardiac uptake of the osteophilic radiopharmaceutical[45].
The accuracy of the scintigraphy performed with osteophilic radiopharmaceutical may be reduced in some cases; in particular, the possibility of obtaining false negative results in patients with ATTRv and phe64leu or val30met mutations with evident cardiac involvement has been described[47,48]; possible false positives have also been described in specific cases of non-infiltrative hypertrophic cardiomyopathies[49,50].
In any case, it is important to remember that osteophilic radiopharmaceuticals are not amyloid-specific and that chemical binding of these radiopharmaceuticals to amyloid deposits remains partially unexplained, although a calcium mediated mechanism has been hypothesized[51].
18F-Sodium Fluoride (18F-NaF) is an excellent PET radiopharmaceutical for bone imaging. Similar to what happens for radiopharmaceuticals used in conventional nuclear medicine, its extraction from the bone is proportional to blood flow and osteoblastic activity, but it is about twice as high as for Tc-99m-based radiotracers[52].
In 2016, Trivieri et al.[53] evaluated seven patients with CA (4 ATTR and 3 AL) and 7 control subjects with a hybrid PET/MR performed using 18F-NaF. They evaluated left-ventricle late gadolinium enhancement (LGE), T1 mapping, and 18F-NaF uptake. All patients with ATTR showed a myocardial uptake of the radiopharmaceutical higher than blood-pool activity, while none of the AL and control subjects showed it. Furthermore, the authors described a correlation between the burden of ATTR on native T1 mapping and
However, some publications on the comparison between 18F-NaF and 99mTc-labeled osteophilic radiopharmaceuticals have shown a lower sensitivity of PET compared to scintigraphy[55,56]. Although this method is more advantageous than osteophilic radiopharmaceutical scintigraphy in dosimetric terms, the significantly higher cost of 18F-NaF and its lower accuracy in identifying ATTR have limited its use to the research field only.
CONCLUSIONS
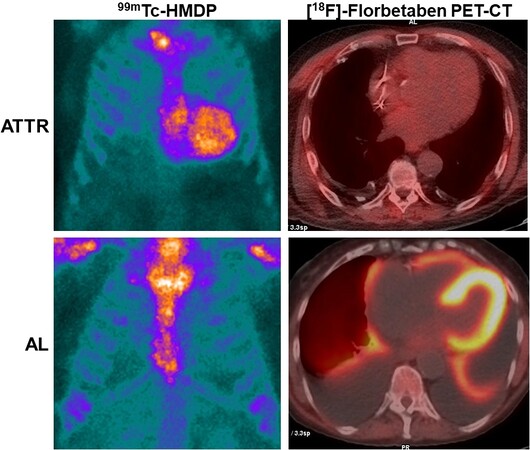
Nuclear medicine offers various research and clinical investigative possibilities in patients with suspected or confirmed AC [Table 1]. The availability of various molecular targets labeled with radioisotopes allows studying the organic alterations caused by the infiltration of amyloidogenic proteins that are reflected in the phenotypic spectrum of the disease. Among all, scintigraphy with osteophilic radiopharmaceuticals has given a substantial contribution to the differential diagnosis of this pathology; unfortunately, scintigraphy with osteophilic radiopharmaceuticals is not able by itself to identify all forms of CA and does not allow excluding an AL form in the case of concomitant monoclonal gammopathy. In this sense, PET/CT with specific radiopharmaceuticals for the amyloid substance could represent an additional non-invasive diagnostic tool, helping to reduce the time required for diagnosis and avoiding the need to use invasive tests with potential serious complications such as endomyocardial biopsy [Figure 2].
Figure 2. Cardiac uptake of PET amyloid tracer compared with the outcome of osteophilic tracer scintigraphy in patients with ATTR and AL.
Main characteristics and differences among the most commonly used radiopharmaceuticals for CA
| Radiopharmaceutical | Activity | Time between injection and acquisition | Image type | Contraindications |
| 99mTc-PYP | 370-740 MBq | 1 h (3 h if needed) | Planar and SPECT (or SPECT-CT) | Pregnancy or breast-feeding |
| 99mTc-DPD | 370-740 MBq | 2-3 h | Planar and SPECT (or SPECT-CT) | Pregnancy or breast-feeding |
| 99mTc-HMDP | 370-740 MBq | 2-3 h | Planar and SPECT (or SPECT-CT) | Pregnancy or breast-feeding |
| 18F-Florbetaben | 111-185 MBq | 1-2 h | Static PET-CT | Pregnancy, breast-feeding, severe renal and/or liver failure |
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and to the writing of this paper: Genovesi D, Giorgetti A
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Both authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
REFERENCES
1. Low YH, Ang ES, Goh AS, Sundram FX, Sin FL. Technetium-99m (Tc-99m) diphosphono-propanedicarboxylic acid bone tracer uptake and Tc99m sestamibi distribution in cardiac amyloidosis - a case report. Ann Acad Med Singapore 1995;24:898-901.
2. Yen TC, Tzen KY, Chen KS, Tsai CJ. The value of gallium-67 and thallium-201 whole-body and single-photon emission tomography images in dialysis-related beta 2-microglobulin amyloid. Eur J Nucl Med 2000;27:56-61.
3. Wechalekar K, Ng FS, Poole-Wilson PA, et al. Cardiac amyloidosis diagnosed incidentally by bone scintigraphy. J Nucl Cardiol 2007;14:750-3.
4. Kodama K, Hamada M, Kuwahara T, et al. Rest-redistribution thallium-201 myocardial scintigraphic study in cardiac amyloidosis. Int J Card Imaging 1999;15:371-8.
5. Hauser W, Atkins HL, Richards P. Amyloidosis of the heart. Possible pitfall in the diagnosis of pericardial effusion by scintillation scanning. JAMA 1968;204:628-30.
6. Gerson MC, Colthar MS, Fowler NO. Differentiation of constrictive percarditis and restrictive cardiomyopathy by radionuclide ventriculography. Am Heart J 1989;118:114-20.
7. Hongo M, Fujii T, Hirayama J, Kinoshita O, Tanaka M, Okubo S. Radionuclide angiographic assessment of left ventricular diastolic filling in amyloid heart disease: a study of patients with familial amyloid polyneuropathy. J Am Coll Cardiol 1989;13:48-53.
8. Clements IP, Olson LJ, Scanlon PD, Gertz MA, Mullany CJ. The effect of respiration on left ventricular diastolic filling as assessed by radionuclide ventriculography. Nucl Med Commun 2000;21:55-63.
9. Arbab AS, Koizumi K, Toyama K, Arai T, Yoshitomi T, Araki T. Scan findings of various myocardial SPECT agents in a case of amyloid polyneuropathy with suspected myocardial involvement. Ann Nucl Med 1997;11:139-41.
10. Ohta H, Endo K, Kanoh T, Konishi J, Kotoura H. Technetium-99m (V) DMSA uptake in amyloidosis. J Nucl Med 1989;30:2049-52.
11. Gazzilli M, Bertoli M, Albano D, et al. Cardiac amyloidosis incidentally detected by 18F-FDG PET/CT. J Nucl Cardiol 2020;27:2429-31.
12. Tanaka H, Hosono M, Kanagaki M, et al. A case of cardiac amyloidosis incidentally detected by bone scintigraphy. Asia Ocean J Nucl Med Biol 2021;9:71-5.
13. Tuominen H, Haarala A, Tikkakoski A, Kähönen M, Nikus K, Sipilä K. 18-FDG-PET in a patient cohort suspected for cardiac sarcoidosis: Right ventricular uptake is associated with pathological uptake in mediastinal lymph nodes. J Nucl Cardiol 2020;27:109-17.
14. Tanaka M, Hongo M, Kinoshita O, et al. Iodine-123 metaiodobenzylguanidine scintigraphic assessment of myocardial sympathetic innervation in patients with familial amyloid polyneuropathy. J Am Coll Cardiol 1997;29:168-74.
15. Hongo M, Urushibata K, Kai R, et al. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J 2002;144:122-9.
16. Lekakis J, Dimopoulos MA, Prassopoulos V, et al. Myocardial adrenergic denervation in patients with primary (AL) amyloidosis. Amyloid 2003;10:117-20.
17. Noordzij W, Glaudemans AW, van Rheenen RW, et al. (123)I-Labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis. Eur J Nucl Med Mol Imaging 2012;39:1609-17.
18. Coutinho MC, Cortez-Dias N, Cantinho G, et al. Reduced myocardial 123-iodine metaiodobenzylguanidine uptake: a prognostic marker in familial amyloid polyneuropathy. Circ Cardiovasc Imaging 2013;6:627-36.
19. Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med 1990;323:508-13.
20. Hazenberg BP, van Rijswijk MH, Piers DA, et al. Diagnostic performance of 123I-labeled serum amyloid P component scintigraphy in patients with amyloidosis. Am J Med 2006;119:355.e15-24.
21. Aprile C, Marinone G, Saponaro R, Bonino C, Merlini G. Cardiac and pleuropulmonary AL amyloid imaging with technetium-99m labelled aprotinin. Eur J Nucl Med 1995;22:1393-401.
22. Schaadt BK, Hendel HW, Gimsing P, Jønsson V, Pedersen H, et al. 99mTc-aprotinin scintigraphy in amyloidosis. J Nucl Med 2003;44:177-83.
23. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306-19.
24. Antoni G, Lubberink M, Estrada S, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med 2013;54:213-20.
25. Choi SR, Golding G, Zhuang Z, et al. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med 2009;50:1887-94.
26. Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). J Nucl Med 2010;51:913-20.
27. Yang L, Rieves D, Ganley C. Brain amyloid imaging--FDA approval of florbetapir F18 injection. N Engl J Med 2012;367:885-7.
28. Dorbala S, Vangala D, Semer J, et al. Imaging cardiac amyloidosis: a pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging 2014;41:1652-62.
29. Vandenberghe R, Van Laere K, Ivanoiu A, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 2010;68:319-29.
30. Lhommel R, Sempoux C, Ivanoiu A, Michaux L, Gerber B. Is 18F-flutemetamol PET/CT able to reveal cardiac amyloidosis? Clin Nucl Med 2014;39:747-9.
31. Dietemann S, Nkoulou R. Amyloid PET imaging in cardiac amyloidosis: a pilot study using 18F-flutemetamol positron emission tomography. Ann Nucl Med 2019;33:624-8.
32. Möckelind S, Axelsson J, Pilebro B, Lindqvist P, Suhr OB, Sundström T. Quantification of cardiac amyloid with [18F]Flutemetamol in patients with V30M hereditary transthyretin amyloidosis. Amyloid 2020;27:191-9.
33. Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid β in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol 2008;7:129-35.
34. Law WP, Wang WY, Moore PT, Mollee PN, Ng AC. Cardiac amyloid imaging with 18F-Florbetaben PET: a pilot study. J Nucl Med 2016;57:1733-9.
35. Kircher M, Ihne S, Brumberg J, et al. Detection of cardiac amyloidosis with 18F-Florbetaben-PET/CT in comparison to echocardiography, cardiac MRI and DPD-scintigraphy. Eur J Nucl Med Mol Imaging 2019;46:1407-16.
36. Genovesi D, Vergaro G, Giorgetti A, et al. [18F]-Florbetaben PET/CT for Differential diagnosis among cardiac immunoglobulin light chain, transthyretin amyloidosis, and mimicking conditions. JACC Cardiovasc Imaging 2021;14:246-55.
38. Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005;46:1076-84.
39. Rapezzi C, Quarta CC, Guidalotti PL, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging 2011;38:470-8.
40. Kristen AV, Haufe S, Schonland SO, et al. Skeletal scintigraphy indicates disease severity of cardiac involvement in patients with senile systemic amyloidosis. Int J Cardiol 2013;164:179-84.
41. Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2011;4:659-70.
42. Falk RH, Lee VW, Rubinow A, Hood WB, Cohen AS. Sensitivity of technetium-99m-pyrophosphate scintigraphy in diagnosing cardiac amyloidosis. Am J Cardiol 1983;51:826-30.
43. Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6:195-201.
44. Cappelli F, Gallini C, Di Mario C, et al. Accuracy of 99mTc-Hydroxymethylene diphosphonate scintigraphy for diagnosis of transthyretin cardiac amyloidosis. J Nucl Cardiol 2019;26:497-504.
45. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404-12.
46. Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol 2019;26:2065-123.
47. Musumeci MB, Cappelli F, Russo D, et al. Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis. JACC Cardiovasc Imaging 2020;13:1314-21.
48. Pilebro B, Suhr OB, Näslund U, Westermark P, Lindqvist P, Sundström T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci 2016;121:17-24.
49. Wakfie-Corieh CG, Ramos López N, Saiz-Pardo Sanz M, Pérez Castejón MJ, Vilacosta I. Not all heart uptakes on 99mTc-DPD scintigraphy are amyloidosis: chloroquine-induced cardiomyopathy. Clin Nucl Med 2021;46:e188-9.
50. Chimenti C, Alfarano M, Maestrini V, et al. False-positive bone scintigraphy denoting transthyretin amyloid in elderly hypertrophic cardiomyopathy. ESC Heart Fail 2021; doi: 10.1002/ehf2.13339.
51. Stats MA, Stone JR. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc Pathol 2016;25:413-7.
52. Segall G, Delbeke D, Stabin MG, et al. SNM. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med 2010;51:1813-20.
53. Trivieri MG, Dweck MR, Abgral R, et al. 18F-Sodium fluoride PET/MR for the assessment of cardiac amyloidosis. J Am Coll Cardiol 2016;68:2712-4.
54. Morgenstern R, Yeh R, Castano A, Maurer MS, Bokhari S. 18Fluorine sodium fluoride positron emission tomography, a potential biomarker of transthyretin cardiac amyloidosis. J Nucl Cardiol 2018;25:1559-67.
55. Ng QKT, Sethi P, Saunders TA, Pampaloni MH, Flavell RR. Discordant findings on 18F-NaF and 99mTc-HDP bone scans in a patient with ATTR cardiac amyloidosis. Clin Nucl Med 2018;43:e89-92.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Genovesi D, Giorgetti A. Nuclear medicine techniques for the diagnosis of cardiac amyloidosis: the state of the art. Vessel Plus 2021;5:50. http://dx.doi.org/10.20517/2574-1209.2021.67
AMA Style
Genovesi D, Giorgetti A. Nuclear medicine techniques for the diagnosis of cardiac amyloidosis: the state of the art. Vessel Plus. 2021; 5: 50. http://dx.doi.org/10.20517/2574-1209.2021.67
Chicago/Turabian Style
Genovesi, Dario, Assuero Giorgetti. 2021. "Nuclear medicine techniques for the diagnosis of cardiac amyloidosis: the state of the art" Vessel Plus. 5: 50. http://dx.doi.org/10.20517/2574-1209.2021.67
ACS Style
Genovesi, D.; Giorgetti A. Nuclear medicine techniques for the diagnosis of cardiac amyloidosis: the state of the art. Vessel Plus. 2021, 5, 50. http://dx.doi.org/10.20517/2574-1209.2021.67
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.