Myocardial surgical revascularization as a subspecialty: to be or not to be, that is the question
Abstract
Over the last few decades, a trend for increased specialization has been observed in all surgical domains. This has been driven by the advancement of medical knowledge and technology and by the realization of a clear association between higher volume of cases and better surgical outcomes. The field of cardiothoracic surgery has followed the same trend, but the most commonly performed operation, coronary artery bypass grafting, is still considered a generalist procedure and does not benefit from recognition as a formal subspecialty. There is robust evidence to support that a positive effect on outcomes can be achieved by both increased volume and better quality of surgical techniques and perioperative protocols. We hypothesize that a structured specialized coronary revascularization program can be initiated in every institution through a strong leadership focused on effective mentorship and training that will achieve the benchmark of less than 1% operative mortality following coronary revascularization. This review article makes a case for recognition of myocardial surgical revascularization as a subspecialty and proposes a strategy to overcome the barriers that preclude such a recognition.
Keywords
BACKGROUND: THE INHERENT NEED FOR SUBSPECIALIZATION
Adopting a more focused approach to training and delivering clinical care through subspecialization has been a constant trend over the last couple of decades. This has been driven by the continuous advancement in knowledge and technology which no longer suits a generalist approach for providing patient care. This is especially true in surgical specialties, where a clear relation between both surgeon-specific and unit-specific volume and patient outcome has been demonstrated, with lower volumes being consistently associated with poorer outcomes across a wide range of complex procedures[1,2]. While surgeon-specific factors contribute significantly to the observed differences, there is an institutional component as well, which is best emphasized in studies looking at failure to rescue following a major postoperative complication, with higher volume centers achieving better results[3].
Therefore, many trainees and young surgeons have naturally adopted the tendency to narrow their focus of practice and embark on specialized training early on in the career through fellowships and early specialization programs. This has been shown to be effective in general surgery where various subspecialization domains are available from colorectal and bariatric to esophageal and hepatobiliary alongside general surgery. Indeed, residents who were enrolled in early specialization programs reported higher satisfaction and greater preparedness for independent practice as well as higher number of cases compared to general pathways of training[4,5]. Similarly, in orthopedic surgery, outcomes and case volumes have been shown to be better if surgeons are fellowship-trained in a certain subspecialty, a result consistent over a wide range of procedures[6,7].
The vast field of cardiothoracic surgery has witnessed a similar trend with the segregation in adult cardiac, thoracic, and congenital cardiac surgery. Moreover, adult cardiac surgery has seen further subspecialization in several domains. One such domain is heart transplantation and mechanical circulatory support that requires a dedicated heart failure team with a specific skillset[8]. Mitral valve surgery is also an area where data from large US registries show a clear relationship between higher individual surgeon case volume and higher likelihood for repair as well as improvements in 30-day and one-year mortality. This remains true when institutional level data are compared[9,10]. In the subspecialized domain of aortic valve repair and root surgery, a similar volume-outcome relationship exists[11,12] and better results have been demonstrated by standardizing practice and techniques in centers with recognized experts in the field[13,14]. Similarly, for Type A aortic dissection, the relationships between higher surgeon and institutional volumes and lower mortality remains valid[15], although significant variation in practice still exists[16]. Furthermore, the emergence of endovascular techniques and therapies in the thoracic aortic domain in the last decade necessitates surgeons to gain specific wire- and catheter-based skills which are not readily acquired by following a generalist training pathway.
Nevertheless, the aforementioned procedures arguably represent only up to a third of cardiac surgical interventions performed worldwide. On the other hand, coronary artery bypass grafting (CABG) remains the commonest cardiac surgical procedure performed globally. In the latest report from the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database, CABG alone accounted for 55% (n = 157,704 cases) of all procedures undertaken[17]. Aortic valve replacement alone or in combination with CABG represented another 15% (n = 41,129) of all procedures. Similarly, in the United Kingdom, CABG comprised 45% of all cardiac surgical procedures (n = 14,527 out of 32,295 cases) according to the National Adult Cardiac Surgery Summary Report published in 2019[18]. Given how much it contributes and influences patients’ outcomes worldwide, it is somewhat surprising that CABG remains unrecognized as a subspecialty and is still viewed as a generalist procedure globally[19].
THE CASE FOR SUBSPECIALIZATION IN SURGICAL MYOCARDIAL REVASCULARIZATION
Surgical myocardial revascularization, with a history spanning more than half a century, has been proven to be a safe and effective procedure for patients with coronary artery disease (CAD)[20]. According to European Society of Cardiology (ESC) Guidelines as well as American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) Guidelines, CABG has a Class I indication for the treatment of left main stem, proximal left anterior descending artery (LAD), or complex three-vessel CAD[21,22]. Additionally, CABG offers a prognostic benefit when compared to percutaneous coronary intervention in patients with diabetes mellitus and in those with low ejection fraction. A recent metanalysis of randomized controlled trials looking at long-term all-cause and cardiac-specific mortality after CABG vs. PCI has shown that PCI was associated with a higher rate of all-cause [incidence rate ratio (IRR) = 1.17; 95%CI: 1.05-1.29] and cardiac-specific mortality (IRR = 1.24; 95%CI: 1.05-1.45) at a mean follow-up of 5.3 years[23].
While CABG has provided reliable results over time, the surgical technique and major complication rates have remained largely unchanged[17,20]. Conversely, the field of interventional cardiology has been constantly evolving with the addition of novel drug-eluting stents, intracoronary imaging, and flow measurement techniques as well as atherectomy devices which have had a clear role in the expansion of PCI possibilities.
There is therefore scope and a clear incentive to improve outcomes following CABG. As with the other subdomains of adult cardiac surgery, a volume-outcome relationship exists for CABG as well[1,2]. However, some authors have argued that volume per se is not a guarantee of better outcomes, but rather, by adhering to quality standards in performing CABG, we can achieve an improvement in survival[24,25]. Therefore, the argument of increased quality rather than quantity has been put forward.
An essential quality metric advocated by professional organizations is the use of multiple arterial grafts as opposed to single internal thoracic artery plus saphenous vein graft[21,26]. This has been shown to improve long-term outcomes in meta-analyses of large observational studies[27,28]. The main reason for the better survival is considered the demonstrated higher long-term patency rates for the additional arterial grafts (radial artery and right internal thoracic artery)[29,30]. However, the largest randomized controlled trial that compared single ITA with bilateral ITA showed no long-term difference in survival in the intention-to-treat analysis, but concerns about the results have been raised as 40% of patients did not receive their allocated treatment[31]. Nevertheless, using more arterial grafts increases procedural complexity and the volume-outcome relationship becomes more important, as shown in a large cohort of patients from the STS database that found lower mortality in centers experienced in using bilateral internal thoracic arteries[32,33]. Although recommended in the practice guidelines, the overall use of multiple arterial grafts remains relatively low (around 5% in the US and 20% in Europe), which can be explained by the higher complexity associated with this technique. In this respect, having an experienced group of surgeons specialized in multiple arterial grafts who can train junior colleagues would positively impact outcomes.
Another potential advantage of using multiple arterial grafts is the possibility to perform off-pump CABG (OPCABG) without manipulation of the ascending aorta (an-aortic OPCABG). This has been shown to reduce the risk of stroke by 78% when compared to conventional on-pump CABG[34]. However, this is arguably the most technically challenging variant of CABG and an analysis of registry data from the US showed that OPCABG has better in-hospital results compared to on-pump surgery for large volume centers and surgeons but worse outcomes when compared to conventional on-pump CABG in low volume settings[35]. This further underlines the point that these techniques should be performed by specialized coronary surgeons.
In addition, minimal-access surgery has expanded in recent years, especially in aortic and mitral valve procedures, driven by a need to offer alternatives to the minimal invasiveness of transcatheter approaches. The field of CABG surgery has not seen a similar transition, but minimal-access, robotic-assisted, and hybrid revascularization techniques have been described. While there are no data from randomized controlled trials, observational studies confirm these methods are safe and effective in selected cases and may positively influence postoperative pain, rate of sternal wound infection, and in-hospital length of stay[36-38]. Although these results may look promising when performed in specialized centers by highly skilled surgeons, their up-take by the wider community has been very low, probably due to the added costs, complexity, and steep learning curve[35,39-42] [Table 1].
Volumes for negotiating learning curve and improving outcomes
| Strategy | Number per surgeon | Number per centre |
| OPCAB | ≥ 48 cases | ≥ 164 cases |
| Minimal access multivessel CABG* | 50 cases | 50-100 cases |
| Robotic CABG* | 35 cases | 50-100 cases |
STRATEGY TO INITIATE RECOGNITION OF SURGICAL MYOCARDIAL REVASCULARIZATION AS A SUBSPECIALTY
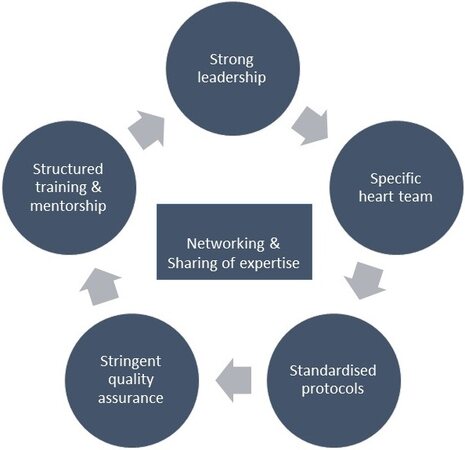
We believe that the strategy to initiate recognition of surgical myocardial revascularization as a subspecialty should be based on a structured approach encompassing leadership changes; standardizing pre-, intra- and post-operative care protocols; and rigorous quality assurance reviews [Figure 1].
Figure 1. Key components of strategy to initiate recognition of surgical myocardial revascularisation as a subspecialty.
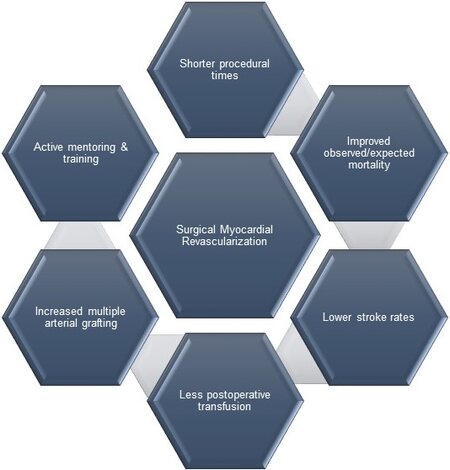
As demonstrated by Watkins et al.[43], these changes can be successfully implemented at an institutional level leading to a consistently improved observed/expected mortality as well as shorter procedural times, lower rates of stroke, and less postoperative transfusion[40]. They achieved this by creating a specialized coronary service within the unit, led by a senior experienced surgeon who actively mentored more junior colleagues. The use of multiple arterial grafts was increased and the surgical techniques were standardized. This led to a reduction in off-pump and robotic procedures, but this in turn made the operations more reproducible and predictable. While the reduction in mortality was significant for elective cases, an even higher improvement was observed in emergent/salvage cases, which underlines the idea that a specialized team is much better in dealing with complex patients and scenarios [Figure 2].
Figure 2. Key performance indicators of a successful surgical myocardial revascularization programme.
Changes to the intraoperative strategy are also paramount and some experts have recommended a staged approach in order to develop a successful program that can adequately manage the higher risk associated with increased complexity. They propose a gradual increase in the use of multiple arterial grafts, starting with the radial artery, progressing to in situ bilateral thoracic arteries, followed by multiple sequential arterial graft arrangements, and finally leading to total anaortic OPCABG[44]. While we believe that no single technique is suitable for all cases, having all of the aforementioned skills available represents an extremely useful approach to providing the best care for the patient. Gaudino et al.[44] also highlighted the importance of intraoperative quality assessment of the grafts with transit-time flow measurement (TTFM). This has been shown to identify grafts needing revision, thus preventing some possible postoperative complications, but no randomized data comparing TTFM vs. no TTFM are currently available[45] [Figure 3].
Figure 3. Pyramidal approach to intraoperative optimization of surgical myocardial revascularization.
Other authors have highlighted the importance of having effective revascularization-specific multidisciplinary heart team meetings focused on all aspects of coronary reinterventions that would select the cases most suitable for surgery[19]. Indeed, it could be argued that one way Watkins et al.[43] managed to improve outcomes was through better patient selection. In addition, having a dedicated coronary surgery center, in which an experienced team could perform a high volume of cases, is very likely to lead to improved outcomes.
The specialized centers can offer structured fellowship programs to residents and trainees who are interested in subspecialty training in surgical myocardial revascularization. The task of professional bodies such as STS, AATS, and EACTS would be to recognize these fellowships as part of a coronary revascularization subspecialty curriculum and to create the networking opportunities that are essential in promoting meaningful mentorship relations.
We believe that this in turn will create research opportunities that will hopefully answer some of the outstanding questions in the field. The Randomized comparison of the clinical Outcome of single vs. Multiple Arterial grafts (ROMA) trial is one such example where collaboration between expert centers will hopefully clarify the question of multiple arterial grafts and their impact on long-term survival[46].
Furthermore, internal reviews and independent audit of the practice should be organized regularly and involve the entire team. During these meetings, results should be compared to desired benchmarks. The 1% operative mortality proposed by leaders in the field[47] and less than 1% postoperative stroke are reasonable targets which would bring CABG on equal levels with PCI. While the real-world results are not currently there yet[48], we believe that through such a focused subspecialized approach this is certainly achievable.
CONS OF RECOGNITION OF SURGICAL MYOCARDIAL REVASCULARIZATION AS A SUBSPECIALTY
The detractors of our hypothesis will contest that usually subspecialities are developed in order to improve patient volume in a few centers and/or for a few surgeons performing infrequent procedures to improve outcome. However, CABG is the bread and butter for every cardiothoracic surgeon as it is the most frequent procedure in cardiac surgery that every senior cardiothoracic surgeon needs to master. If CABG is specialized to certain centers or surgeons with other surgeons not performing it at all or performing it infrequently, then these surgeons will have difficulties when faced with a situation needing acute CABG. This situation is usually encountered during valve surgery or dissections, where a coronary artery stenosis is not known before the start of the operation, e.g., a coronary artery is obstructed due to extension of dissection, embolus, or a kink in a reimplanted coronary button. A corollary of this will be the impact on logistics and on call commitments if CABG is restricted to a few centers or surgeons. Additionally, the recently published SWEDEHEART Annual Report will be cited by some to suggest that at least one country has already achieved the benchmark outcomes without setting up specialized centers for CABG or restricting it to few surgeons. According to this report, in 2019, the 30-day mortality for CABG in Sweden was 0.66% (95%CI: 0.34-0.9) and stroke rate after CABG was 1.0 (95%CI: 0.63-1.4)[49].
Our counter argument is very simple. We are not at all suggesting that CABG be restricted to specialized revascularization centers. Instead, we are hypothesizing that all cardiac surgery centers embrace the concept of subspecialty training in CABG. This means that conventional on-pump low risk CABG remains a generalist procedure and all surgeons perform it. However, certain surgeons in each center should receive structured training, preferably in the final year of their training and early years of independent practice, to learn the full spectrum of surgical techniques for myocardial revascularization. This will allow every center to have specialists in revascularization who are capable of performing the full breadth of surgical myocardial revascularization from conventional on-pump CABG to multivessel OPCABG and robotic CABG. This will enable in-house tackling of infrequent yet challenging situations such as porcelain aorta and surgery for high-risk subgroups. The ethos of recognition of surgical myocardial revascularization as a subspecialty is to produce expert surgeons in every center who are able to offer state-of-the-art surgical myocardial revascularization without compromising the benchmark outcomes.
In conclusion, achieving these goals and results is challenging and developing a truly focused coronary surgery subspecialty will likely take many years, but we have the evidence to support the need for it, and, through efficient networking, strong leadership, and sharing of expertise, we will certainly improve the quality of the care we provide to our patients and advance the field of myocardial surgical revascularization.
DECLARATIONS
Authors’ contributionsMade substantial contributions to the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content, and approval of the final version: Gradinariu G, Raja SG
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117-27.
2. Sahni NR, Dalton M, Cutler DM, Birkmeyer JD, Chandra A. Surgeon specialization and operative mortality in United States: retrospective analysis. BMJ 2016;354:i3571.
3. Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume-outcome effect in cardiovascular surgery: the role of failure to rescue. JAMA Surg 2014;149:119-23.
4. Marcadis AR, Spencer T, Sleeman D, Velazquez OC, Lew JI. Case distributions in general surgery residency: Subspecialization occurs before fellowship. Surgery 2020;167:717-23.
5. Klingensmith ME, Potts JR, Merrill WH, et al. Surgical training and the early specialization program: Analysis of a National Program. J Am Coll Surg 2016;222:410-6.
6. Pour AE, Bradbury TL, Horst P, Harrast JJ, Erens GA, Roberson JR. Trends in primary and revision knee arthroplasty among orthopaedic surgeons who take the American Board of Orthopaedics part II exam. Int Orthop 2016;40:2061-7.
7. Gombera MM, Laughlin MS, Vidal EA, et al. The impact of fellowship type on trends and complications following total shoulder arthroplasty for osteoarthrosis by recently trained board-eligible orthopedic surgeons. J Shoulder Elbow Surg 2020;29:e279-86.
8. Masters J, Morton G, Anton I, et al. Specialist intervention is associated with improved patient outcomes in patients with decompensated heart failure: evaluation of the impact of a multidisciplinary inpatient heart failure team. Open Heart 2017;4:e000547.
9. Chikwe J, Toyoda N, Anyanwu AC, et al. Relation of mitral valve surgery volume to repair rate, durability, and survival. J Am Coll Cardiol ;2017:2397-406.
10. Badhwar V, Vemulapalli S, Mack MA, et al. Volume-outcome association of mitral valve surgery in the United States. JAMA Cardiol ;2020:1092.
11. Bilkhu R, Youssefi P, Soppa G, et al. Aortic root surgery: Does high surgical volume and a consistent perioperative approach improve outcome? Semin Thorac Cardiovasc Surg 2016;28:302-9.
12. Hughes GC, Zhao Y, Rankin JS, et al. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg 2013;145:166-70.
13. Ehrlich T, de Kerchove L, Vojacek J, et al. State-of-the art bicuspid aortic valve repair in 2020. Prog Cardiovasc Dis 2020;63:457-64.
14. Mastrobuoni S, de Kerchove L, Navarra E, et al. Long-term experience with valve-sparing reimplantation technique for the treatment of aortic aneurysm and aortic regurgitation. J Thorac Cardiovasc Surg 2019;158:14-23.
15. Chikwe J, Cavallaro P, Itagaki S, Seigerman M, Diluozzo G, Adams DH. National outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thorac Surg 2013;95:1563-9.
16. Mazzeffi M, Ghoreishi M, Alejo D, et al. Investigators for the Maryland Cardiac Surgery Quality Initiative. Clinical practice variation and outcomes for Stanford Type A aortic dissection repair surgery in Maryland: Report from a statewide quality initiative. Aorta (Stamford) 2020;8:66-73.
17. Bowdish ME, D’Agostino RS, Thourani VH, et al. The society of thoracic surgeons adult cardiac surgery database: 2020 update on outcomes and research. Ann Thorac Surg 2020;109:1646-55.
18. . NICOR. National adult cardiac surgery audit. 2019 summary report (2015/16-2017/18 data). Available from national-adult-cardiac-surgery-summary-report-2019-final.pdf (hqip.org.uk). [Last accessed on 8 Feb 2021]
19. Mack M, Taggart D. Coronary revascularization should be a subspecialty focus in cardiac surgery. J Thorac Cardiovasc Surg 2019;157:945-7.
21. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165.
22. Hillis LD, Smith PK, Anderson JL, et al. American College of Cardiology Foundation. 2011 ACCF/AHA Guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;58:e123-210.
23. Gaudino M, Hameed I, Farkouh ME, et al. Overall and cause-specific mortality in randomized clinical trials comparing percutaneous interventions with coronary bypass surgery: a meta-analysis. JAMA Intern Med 2020;180:1638-46.
24. Welke KF, Barnett MJ, Sarrazin MS, Rosenthal GE. Limitations of hospital volume as a measure of quality of care for coronary artery bypass graft surgery. Ann Thorac Surg 2005;80:2114-9.
25. Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg 2012;143:287-93.
26. Aldea GS, Bakaeen FG, Pal J, et al. Society of Thoracic Surgeons. The Society of Thoracic Surgeons Clinical Practice Guidelines on arterial conduits for coronary artery bypass grafting. Ann Thorac Surg 2016;101:801-9.
27. Taggart DP, D’amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001;358:870-5.
28. Yi G, Shine B, Rehman SM, Altman DG, Taggart DP. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation 2014;130:539-45.
29. Gaudino M, Benedetto U, Fremes S, et al. RADIAL Investigators. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N Engl J Med 2018;378:2069-77.
30. Buxton BF, Hayward PA, Raman J, et al. RAPCO Investigators*. Long-term results of the RAPCO trials. Circulation 2020;142:1330-8.
31. Taggart DP, Benedetto U, Gerry S, et al. Arterial Revascularization Trial Investigators. Bilateral versus single internal-thoracic-artery grafts at 10 years. N Engl J Med 2019;380:437-46.
32. Schwann TA, Habib RH, Wallace A, et al. Bilateral internal thoracic artery versus radial artery multi-arterial bypass grafting: a report from the STS database†. Eur J Cardiothorac Surg 2019;56:926-34.
33. Schwann TA, Tatoulis J, Puskas J, et al. Worldwide trends in multi-arterial coronary artery bypass grafting surgery 2004-2014: a tale of 2 continents. Semin Thorac Cardiovasc Surg 2017;29:273-80.
34. Zhao DF, Edelman JJ, Seco M, et al. Coronary artery bypass grafting with and without manipulation of the ascending aorta: a network meta-analysis. J Am Coll Cardiol 2017;69:924-36.
35. Benedetto U, Lau C, Caputo M, et al. Comparison of outcomes for off-pump versus on-pump coronary artery bypass grafting in low-volume and high-volume centers and by low-volume and high-volume surgeons. Am J Cardiol 2018;121:552-7.
36. Ruel M, Shariff MA, Lapierre H, et al. Results of the minimally invasive coronary artery bypass grafting angiographic patency study. J Thorac Cardiovasc Surg 2014;147:203-8.
37. Kikuchi K, Mori M. Minimally invasive coronary artery bypass grafting: a systematic review. Asian Cardiovasc Thorac Ann 2017;25:364-70.
38. Hammal F, Nagase F, Menon D, Ali I, Nagendran J, Stafinski T. Robot-assisted coronary artery bypass surgery: a systematic review and meta-analysis of comparative studies. Can J Surg 2020;63:E491-508.
39. Ravikumar N, George V, Shirke MM, Ashry A, Harky A. Robotic coronary artery surgery: outcomes and pitfalls. J Card Surg 2020;35:3108-15.
40. McGinn JT Jr, Usman S, Lapierre H, Pothula VR, Mesana TG, Ruel M. Minimally invasive coronary artery bypass grafting: dual-center experience in 450 consecutive patients. Circulation 2009;120:S78-84.
41. Amabile A, Torregrossa G, Balkhy HH. Robotic-assisted coronary artery bypass grafting: current knowledge and future perspectives. Minerva Cardioangiol 2020;68:497-510.
42. Bonatti J, Schachner T, Bernecker O, et al. Robotic totally endoscopic coronary artery bypass: program development and learning curve issues. J Thorac Cardiovasc Surg 2004;127:504-10.
43. Watkins AC, Ghoreishi M, Maassel NL, et al. Programmatic and surgeon specialization improves mortality in isolated coronary bypass grafting. Ann Thorac Surg 2018;106:1150-8.
44. Gaudino MFL, Sandner S, Bonalumi G, Lawton JS, Fremes SE. Coronary Task Force of the European Association for Cardio-Thoracic Surgery. How to build a multi-arterial coronary artery bypass programme: a stepwise approach. Eur J Cardiothorac Surg 2020;58:1111-7.
45. Thuijs DJFM, Bekker MWA, Taggart DP, et al. Improving coronary artery bypass grafting: a systematic review and meta-analysis on the impact of adopting transit-time flow measurement. Eur J Cardiothorac Surg 2019;56:654-63.
46. Gaudino MFL, Taggart DP, Fremes SE. The ROMA trial: why it is needed. Curr Opin Cardiol 2018;33:622-6.
48. LaPar DJ, Filardo G, Crosby IK, et al. The challenge of achieving 1% operative mortality for coronary artery bypass grafting: a multi-institution Society of Thoracic Surgeons Database analysis. J Thorac Cardiovasc Surg 2014;148:2686-96.
49. Årsrapport 2019 - UCR (SWEDEHEART Annual Report 2019). Available from https://www.ucr.uu.se/swedeheart/dokument-sh/arsrapporter-sh. [Last accessed on 8 Feb 2021].
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Gradinariu G, Raja SG. Myocardial surgical revascularization as a subspecialty: to be or not to be, that is the question. Vessel Plus 2021;5:22. http://dx.doi.org/10.20517/2574-1209.2020.86
AMA Style
Gradinariu G, Raja SG. Myocardial surgical revascularization as a subspecialty: to be or not to be, that is the question. Vessel Plus. 2021; 5: 22. http://dx.doi.org/10.20517/2574-1209.2020.86
Chicago/Turabian Style
Gradinariu, George, Shahzad G. Raja. 2021. "Myocardial surgical revascularization as a subspecialty: to be or not to be, that is the question" Vessel Plus. 5: 22. http://dx.doi.org/10.20517/2574-1209.2020.86
ACS Style
Gradinariu, G.; Raja SG. Myocardial surgical revascularization as a subspecialty: to be or not to be, that is the question. Vessel Plus. 2021, 5, 22. http://dx.doi.org/10.20517/2574-1209.2020.86
About This Article
Copyright
Data & Comments
Data

 Cite This Article 6 clicks
Cite This Article 6 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.