Routine use of cerebral protection devices during transcatheter aortic valve implantation: what does the evidence say?
Abstract
Transcatheter aortic valve implantation (TAVI) is a well-established treatment for symptomatic severe aortic stenosis in intermediate and high-risk patients. However, as TAVI indications increase, concerns regarding adverse events and complications rise in the same proportion. Stroke is one of the most feared TAVI complications and a hard endpoint present in all TAVI studies. TAVI-related stroke incidence becomes even more relevant with TAVI indications spreading to younger, low/intermediate-risk patients. Several devices have been developed to prevent this catastrophic event, some of them being broadly used. Nevertheless, the evidence for routine use of cerebral embolic protection devices is still controversial.
Keywords
Introduction
Transcatheter aortic valve implantation (TAVI) is a well-established and widespread option to treat severe aortic stenosis in intermediate and high-risk patients. However, its increased use brings some intrinsic concerns, especially in low-risk and younger patients, a population in which even a low rate of adverse events can be catastrophic[1-7]. In this setting, to reduce or, ideally, to eliminate neurological complications is especially relevant.
To mitigate neurological events risk, cerebral embolic protection devices (CEPD) were developed aiming to capture embolized debris and/or to prevent them from reaching the cerebral circulation. Even with some evidence supporting CEPD benefits and safety, the lack of a single randomized clinical trial demonstrating reduction in hard outcomes, such as stroke and mortality, has limited the widespread acceptance of CEPD and its routine use.
This article offers an updated state-of-the-art review on CEPD use and which patient profile is most likely to benefit from this therapy.
Issue relevance
The incidence of clinically relevant neurological events after TAVI varies from 1 to 10%, but it can be as high as 94% if silent events detected by brain imaging are also considered[8]. The majority of post-TAVI strokes have an embolic origin and occur in the early post-TAVI period (64% and 85% at 2 and 7 days, respectively). These are referred to as procedure-related neurological events.
Calcium debris embolization can happen during catheter and wire manipulation, valve implantation, pre-dilatation, and/or post-dilatation[2,5]. Compared to native valves, valve-in-valve and bicuspid aortic valve are associated with higher stroke rates due to the need for increased valve manipulation or the presence of highly calcified anatomies. Debris embolization can also be secondary to small thrombus formation or embolization from atherosclerotic plaques in the ascending aorta and aortic arch.
Regarding the clinical relevance, patients who suffer a stroke are at high risk for mortality and severe morbidity including physical disability[9,10]. In a meta-analysis conducted by Eggebrecht et al.[11], patients with cerebrovascular events presented a 3.5-fold higher 30-day mortality than those without events (25.5% vs. 6.9%, respectively). In another study, short- and long-term mortality risks were incremental according to cerebrovascular events severity, with a significantly higher mortality rates in the presence of major stroke [30-day mortality: odds ratio (OR) = 7.43; 95% confidence interval (CI): 2.45-22.53; P = 0.001, late mortality: hazard ratio (HR) = 1.75; 95%CI: 1.01-3.04; P = 0.043][12]. Similarly, a meta-analysis of 29,034 patients showed a 30-day mortality following stroke of 12.27%, with stroke-related mortality of 28.22%, compared with 6.4% mortality in patients without a stroke (OR = 6.45; 95%CI: 3.9-10.66; P < 0.0001)[13]. Furthermore, it is valid to emphasize that 30-day permanent disability is found in around 50% of patients who have suffered a stroke[14] and that even silent cerebral emboli are associated with worse outcomes, three times higher risk of clinical stroke, two times higher risk of dementia and declined cognitive function.
Notwithstanding, cerebrovascular events present a high impact on patient’s quality of life, a consequence even more feared than death. Interesting research showed that, in terms of postoperative perspectives, the majority of patients undergoing TAVI had as their primary objective the maintenance of their independence and being able to practice daily hobbies, but only 7% had staying alive after the procedure as their main goal[15]. These results highlight the importance of patients’ quality of life as endpoint, which should be considered during the TAVI decision-making process.
Cerebral protection system
Recent data suggested that CEPD use is associated with less overt strokes, lower total lesion volume, and a smaller number of new ischemic lesions detected by post-procedural magnetic resonance imaging (MRI) studies[10,16-19].
So far, several CEPD have been developed by many manufactures, including ProtEmbo, Sentinel, TriGUARD, Emblok, Emboline, Embrella, and Embol-X[20-22]. They vary not only in the mechanism for protection, for instance, capture versus deflection, but also in the access site and delivery sheath size [Table 1]. However, only the Sentinel is already approved by the Food and Drug Administration (FDA), being the most used and studied device. A summary of the current published and ongoing trials regarding cerebral protection during TAVI is presented in Table 2.
Main cerebral protection devices
| Coverage | Access site | Delivery sheath | Pore size | Mechanism | |
|---|---|---|---|---|---|
| Sentinel | BCT, LCCA | Radial | 6F | 140 μm | Capture |
| TriGUARD | Full arch | Femoral | 8F | 115 × 145 μm | Deflection |
| Embrella | BCT, LCCA | Radial | 6F | 100 μm | Deflection |
| ProtEmbo | Full arch | Radial | 6F | 60 μm | Deflection |
| Emblok | Full arch | Femoral | 11F | 125 μm | Capture |
| Embol-X | Full body | Transaortic | 17F | 120 μm | Capture |
| Emboliner | Full body | Femoral | 9F | 150 μm | Capture |
Main trials evaluating the use of cerebral embolic protection devices during TAVI
| Trial | Year of publication | Device studied | Study design | Endpoints | Population | Main results (device vs. no device) |
|---|---|---|---|---|---|---|
| Published trials | ||||||
| MISTRAL-C | 2016 | Sentinel (CE mark and FDA approval) | Randomized clinical trial | Primary endpoint: new cerebral lesions by DW-MRI 5 to 7 days after TAVI | From January 2013 to July 2015, 65 patients randomized 1:1 to transfemoral TAVI with or without Sentinel | Device success: 93%
New brain lesions: 78% Absence of new lesions: 13% vs. 27%; P = 0.31 Total lesion volume: 95 mm3 (10-257) vs. 197 mm3 (95-525) ≥ 10 new brain lesions: 0 vs. 20%; P = 0.03 Neurocognitive deterioration: 4% vs. 27%; P = 0.017 |
| CLEAN-TAVI | 2016 | Sentinel | Randomized clinical trial | Primary endpoint: numerical difference in new positive postprocedure DW-MRI brain lesions at 2 days after TAVI in potentially protected territories.
Secondary outcome: difference in volume of new lesions after TAVI in potentially protected territories | From April 2013 to June 2014, 100 patients randomized 1:1 to TAVI with or without Sentinel | Device success: 92%
New cerebral lesions: 98% Number of new lesions: 4 (3-7.25) vs. 10 (6.75-17); P < 0.001 New lesion volume: 242 mm3 (159-353) vs. 527 mm3 (364-830); P = 0.001 Stroke incidence: 10% vs. 10% |
| SENTINEL trial | 2017 | Sentinel | Randomized clinical trial | Primary safety endpoint: MACCE at 30 days
Primary efficacy endpoint: reduction in new lesion volume in protected brain territories by MRI at 2 to 7 days after TAVI | 19 centers
363 patients randomized 2:1 to TAVI with or without Sentinel | Device success: 94.4%
Debris found within filters: 99% MACCE: 7.3% vs. 9.9%; P = 0.41 New lesion volume: 102.8 mm3vs. 178 mm3; P = 0.33 30 days stroke: 5.6% vs. 9.1%; P = 0.25 |
| DEFLECT I | 2015 | TriGuard
(CE mark approval; applied for FDA approval) | Prospective, multicentre trial | Primary safety endpoint: in-hospital device- or procedure-related cardiovascular mortality, major stroke disability, life-threatening bleeding, distal embolisation, major vascular complications, or need for acute cardiac surgery | 37 consecutive patients undergoing TAVI with the TriGuard | Successful cerebral coverage: 80%
Primary outcome: 8.1% New cerebral ischaemic lesions on post-procedure DW-MRI: 82% Per-patient total lesion volume: 34% lower than historical cohorts (0.2 cm3vs. 0.3 cm3) |
| DEFLECT III | 2015 | TriGuard | Randomized clinical trial | Primary endpoint: in-hospital procedural safety (death, stroke, life-threatening or disabling bleeding, stage 2/3 acute kidney injury, or major vascular complications)
Secondary device performance endpoint: technical success (successful device deployment, positioning with complete three-vessel coverage) | 13 centers in Europe and Israel
From February 2014 to March 2015, 85 patients randomized to TAVI with (n = 46) or without TriGuard (n = 39) | Primary endpoint: 21.7% vs. 30.8%; P = 0.34
Technical success: 88.9% Per Treatment population (subjects with complete three-vessel cerebral coverage): new ischaemic brain lesions 26.9 vs. 11.5%); new neurologic deficits 3.1 vs. 15.4% |
| REFLECT II | 2020 | TriGuard 3 | Randomized clinical trial | Primary safety endpoint (composite of all-cause mortality, stroke, life-threatening or disabling bleeding, stage 2/3 acute kidney injury, coronary artery obstruction requiring intervention, major vascular complication, and valve-related dysfunction requiring intervention at 30 days
Primary efficacy endpoint (composite of all-cause mortality or stroke at 30 days, National Institute of Health Stroke Scale worsening, absence of DWI-MRI lesions post-procedure, and total volume of cerebral lesions by DWI) | 25 US centers
295 patients randomized 2:1 to TAVI with or without TriGuard 3 | Device successful deployment: 100%
Technical success: 71% Primary safety endpoint: 15.9% device vs. 34.4% performance goal; P non-inferiority = 0.0001 Primary efficacy endpoint: 45.7% vs. 54.3%; P = 0.857 Median total lesion volume: 215.39 mm3vs. 188.09 mm3; P = 0.405 |
| PROTAVI-C Pilot | 2014 | Embrella
(CE Mark approval) | Prospective and nonrandomized trial | Periprocedural cerebral lesions assessed by DW-MRI | 52 patients who underwent transfemoral TAVI with (n = 41) or without (n = 11) Embrella | Device successfully deployed: 100%
7 days DW-MRI new ischemic lesions: 100% vs. 100% Median number of defects per patient: 8 (3-13) vs. 4 (2-8); P = 0.41 Lesion volume per lesion: 30 mm3 (20-50) vs. 50 mm3 (30-70); P = 0.003 |
| EMBOL-X trial | 2015 | EMBOL-X | Prospective, single-center, randomized-controlled trial | Periprocedural cerebral lesions assessed by DW-MRI at baseline and within 7 days post-TAVI | From July 2012 to April 2014, 30 patients randomized 1:1 to TAVI with (n = 14) or without EMBOL-X (n = 16) | New foci of restricted diffusion: 57% vs. 67%; P = 0.7
Lesion size: 88 ± 60 mm3vs. 168 ± 217 mm3; P = 0.27 Lesion volumes in the supply region of the middle cerebral artery: 33 ± 29 mm3vs. 76 ± 67 mm3; P = 0.04 |
| Emblok Embolic study | 2020 | Emblok | Prospective, nonrandomized, multicenter, first-in-man pilot study | Primary endpoint: technical success and immediate cerebral embolic burden after TAVI (number and volume of new brain lesions detected by DW-MRI at days 2 to 5 post-TAVI compared with baseline) | 20 patients submitted to TAVI with Emblok | Device successfully positioned: 100%
Significant debris capture: 90% 30-day MACCE: 0% New ischemic defect post-procedural DW-MRI: 95% Median number of new lesions per patient: 10 (4.75-15.2) Total new lesion volume: 199.9 mm3 (83.9-447.5) Mean lesion volume per lesion: 42.5 mm3 (21.5-75.6) |
| SafePass 2 | Early clinical results presented at TCT 2019 | Emboliner | Prospective, non-randomised, multicentre, open-label study | Primary safety endpoint: 30-day MACCE incidence
Technical performance: technical success (ability to successfully access the aortic arch, position the device and retrieve and remove it) | 3 centers in New Zealand
24 patients submitted to TAVI with Emboliner | Primary safety endpoint: 0%
Primary technical performance endpoint: 100% Debris capture: 100% |
| Ongoing trials | ||||||
| PROTECTED TAVR | Recruiting NCT04149535 | Sentinel | Prospective randomized trial | Primary endpoints :
rate of stroke through 72 hours post-TAVR or discharge (whichever comes first) | Estimated enrollment: 3000 patients randomized to TAVR with or without Sentinel | |
| PROTECT TAVI | Recruiting
NCT02895737 | Sentinel | Prospective randomized trial | Primary endpoint: total new lesion volume in protected brain regions detected by MRI
Secondary endpoint: number of new cerebral lesions detected by MRI; occurrence of clinical stroke and/or neurocognitive dysfunction; postoperative outcome according to VARC 2 criteria | Estimated enrollment: 328 patients submitted to TAVI with or without Sentinel | |
| PROTEMBO SF Trial | Recruitment completed, not published
NCT03325283 | ProtEmbo | Prospective, observational, multi-center, intention-to-treat study | Primary endpoint: procedural success (successful access, delivery to, deployment within, and retrieval of the ProtEmbo System from the aortic arch, adequate coverage of side branch vessels and maintenance of position for duration of the TAVR procedure)
In-hospital procedural safety up to 7 days (MACCEs, including device-related safety outcomes) Stroke severity at 3 and 30 days Occurrence of Serious Adverse Events at 3 and 30 days | Original estimated enrollment: 10 patients submitted to TAVI with ProtEmb | |
Sentinel CPSR [Claret Medical (Boston Scientific, Corp, USA)]
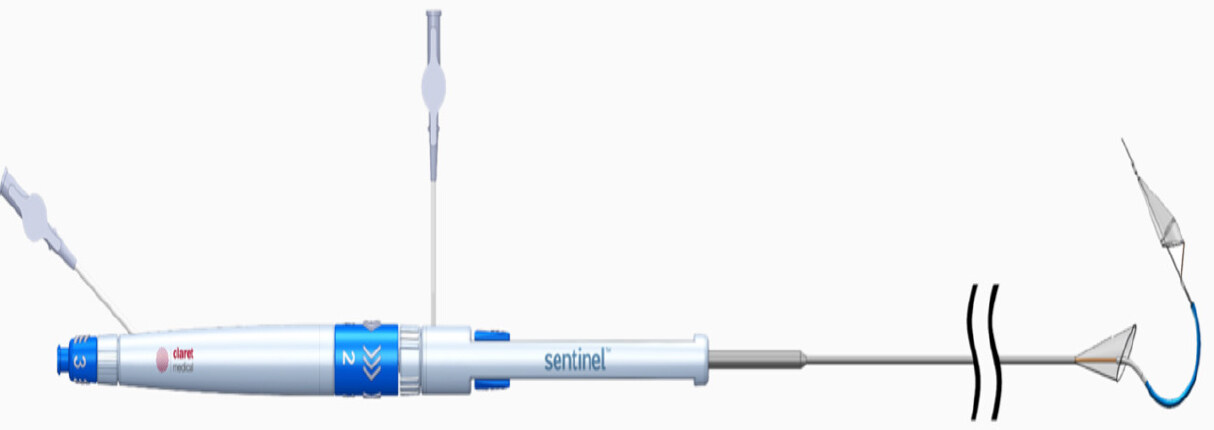
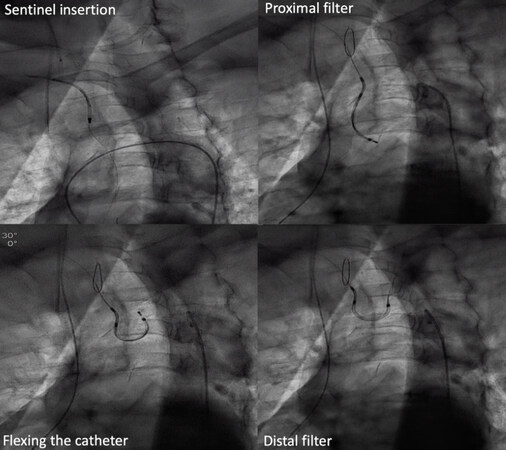
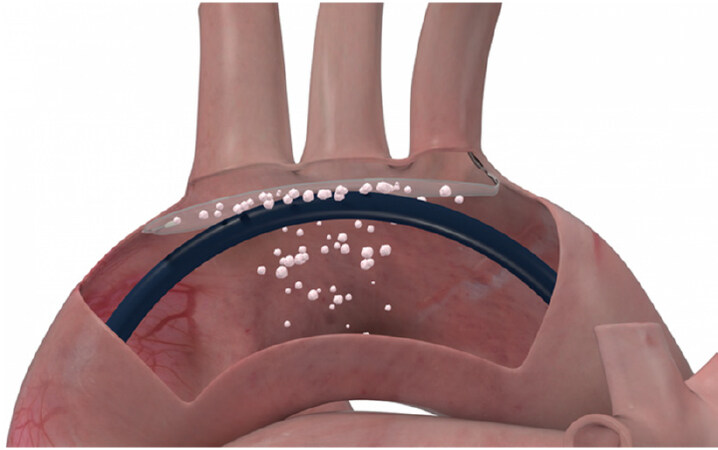
The Sentinel CPS is the most studied cerebral protection device. It is made of 2 inter-connected filters deployed into the brachiocephalic trunk and left common carotid artery through a 6 French size sheath[23]. The most commonly used access is the right radial artery [Figures 1 and 2][18].
Figure 1. Sentinel cerebral protection system. Image provided courtesy of Boston Scientific. © 2020 Boston Scientific Corporation or its affiliates. All rights reserved
Three randomized clinical trials (RCT) evaluating the Sentinel’s role during TAVI were published in 2016, the MISTRAL-C, the CLEAN-TAVI, and the SENTINEL trial[22,24,25]. These trials demonstrated device’s safety and suggested that Sentinel was associated with fewer and smaller brain lesions on postoperative MRI than unprotected TAVIs.
The MISTRAL-C was the first study to enroll 65 TAVI patients submitted to a protected or unprotected TAVI procedure. New brain lesions on MRI studies were found in 78% of patients, with fewer new lesions number (73% vs. 87%; P = 0.31) and total lesion volume [95 mm3 (IQR 10-257) vs. 197 mm3 (95-525); P = 0.171] in the protected group. Ten or more new brain lesions were found only in the control cohort (0% vs. 20%; P = 0.03), and neurocognitive deterioration was present in 4% of patients with received Sentinel during TAVI vs. 27% in those who did not (P = 0.017)[24]. Similarly, the CLEAN-TAVI study randomized 100 patients in 1:1 fashion to TAVI with or without Sentinel insertion. Post-procedure MRI revealed new cerebral lesions in 98% of patients, with a significant smaller new lesion volume [242 mm3 (95%CI: 159-353) vs. 527 mm3 (95% CI 364-830); P = 0.001] and lower number of new lesions two days post-TAVI [4.0 (IQR: 3.00-7.25) vs. 10.0 (IQR 6.75-17.00); P < 0.001] in the Sentinel group. These neuro-imaging differences, however, were not translated into a significant reduction in clinical stroke incidence (10% in each group)[22]. The randomized SENTINEL trial, by its time, included 363 patients with a 2:1 randomization for CEPD vs. no CEPD. Although statistical significance was not achieved, the study demonstrated a strong trend toward stroke reduction within 72 h post-TAVI in the CEPD group compared to the unprotected group (3.0% vs. 8.2%; P = 0.053)[25].
Regardless of the fact that none of these trials have, individually, demonstrated superiority in terms of hard outcomes, such as stroke and mortality, recent meta-analyzes showed that CEPD use was associated with lower rates of stroke and 30-day mortality[18,23]. A propensity-matched patient cohort including 533 patients also showed lower rates of procedural all-stroke (1.88% vs. 5.44%, OR = 0.35, 95%CI: 0.17-0.72, relative risk reduction 65%; P = 0.0028), and the combined endpoint of all-cause mortality and all-stroke (2.06% vs. 6.00%, OR = 0.34, 95%CI: 0.17-0.68, relative risk reduction 66%; P = 0.0013) in the protected TAVI group. The rate of disabling stroke was also substantially lower in the Sentinel group (0.38% vs. 2.44%; P = 0.0045)[26].
Furthermore, in the last months, evidence from two large US databases has suggested that Sentinel use during TAVI was associated with statistically significant reduction in stroke risk. In the Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) TVT Registry, the rate of in-hospital stroke was not significantly lower when the Sentinel device was used according to an instrumental-variable analysis (1.39% vs. 1.54%; RR = 0.90; 95%CI: 0.68-1.13). A secondary propensity-weighted analysis of the data, however, indicated that cerebral protection was associated with a reduction in the rates of in-hospital stroke (1.30% vs. 1.58%; RR = 0.82; 95%CI: 0.69-0.97), in-hospital death or stroke (2.1% vs. 2.5%; RR = 0.84; 95%CI: 0.73-0.98), 30-day stroke (1.9% vs. 2.2%; RR = 0.85; 95%CI: 0.73-0.99), and 30-day death (1.7% vs. 2.2%; RR = 0.78; 95%CI: 0.64-0.95)[27]. Corroborating these findings, a propensity-weighted analysis of the National Inpatient Sample showed that Sentinel use was associated with a lower risk of in-hospital ischemic stroke (1.0% vs. 3.8%; OR = 0.24; 95%CI: 0.09-0.62) and in-hospital death (0% vs. 1%; P = 0.036)[28].
Despite the aforementioned, it is essential to remember that the Sentinel does not protect the left vertebral artery since it is a branch of the left subclavian artery. There are still concerns about leaving the left vertebral artery unprotected, thus some companies are developing devices to eliminate this blind spot.
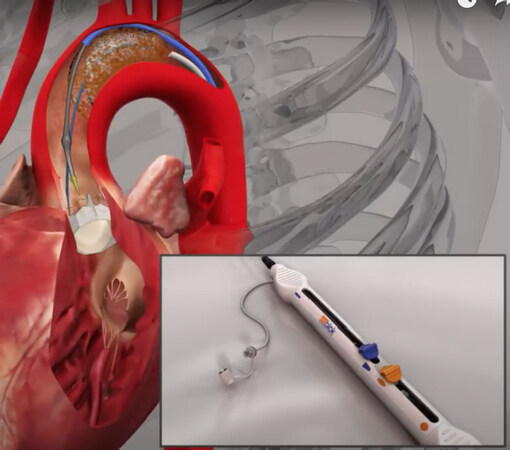
TriGUARDTM (Keystone Heart, Herzliya, Israel)
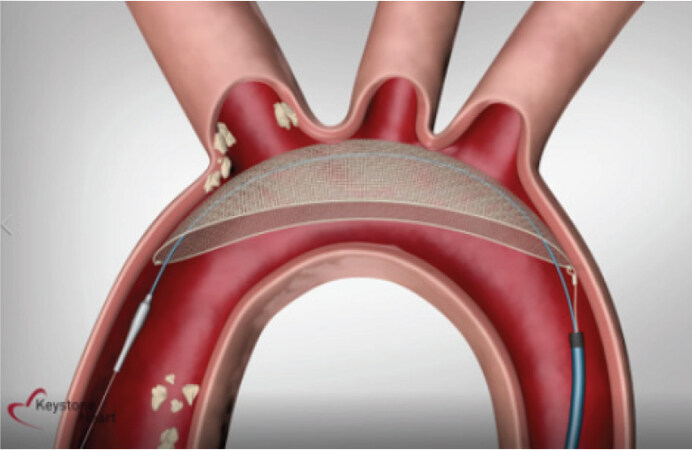
The TriGUARD is the only CE mark approved system designed to cover and protect all three major cerebral aortic arch vessels [Figure 3]. Currently, the device is only in investigational use in the US, planning to apply for FDA approval.
The TriGUARD is inserted through a transfemoral 8F sheath, via the femoral artery access already in use during TAVI, usually at the pigtail insertion side, thus eliminating the need for a third arterial puncture. The device is made of nitinol and consists of a self-positioning, self-stabilizing polymetric mesh with pore sizes 115 × 145 micrometers (μm) opened in the aortic arch, covering the three aortic arch vessels[29].
There are clinical trials already published showing the efficacy and safety of the device. The DEFLECT I and DEFLECT III trial demonstrated that the technical success, which included complete 3-vessel cerebral coverage, was achieved in 80%-90% of the patients. The DEFLECT III demonstrated that this device use is associated with greater freedom from new ischemic brain lesions, fewer new neurologic deficits, and better performance on a delayed memory task at hospital discharge[30,31]. From DEFLECT I to DEFLECT III study, the device has changed from a 250 μm to 130 μm pore size. The REFLECT trial is another randomized clinical trial with larger population, designed to study the TriGUARD 3 device. The TriGUARD 3 is the new generation device, designed to bring some improvements such as a simplified frame design, which eliminates the need for a dedicated stabiliser. It is fully visible via fluoroscopy, contains a reduced filter mesh pore size for deflection of smaller particles (145 µm × 115 µm vs. 250 µm × 250 µm), and has a refined delivery system that reduced the delivery profile (8F instead of 9F)[32]. Recently, Jeffrey W. Moses presented the results from the Reflect II trial during a late-breaking trial session at TCT Connect 2020. The results showed the safety of the TriGUARD 3, but did not demonstrate superiority for the primary hierarchical efficacy endpoint[33].
Embrella Embolic Deflector System (Edwards Lifesciences, Irvine, CA, USA)
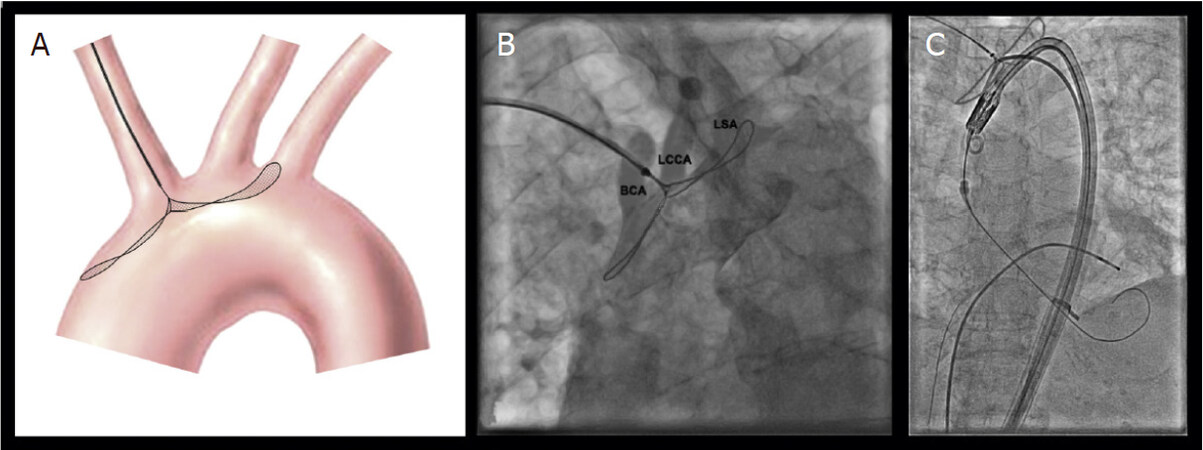
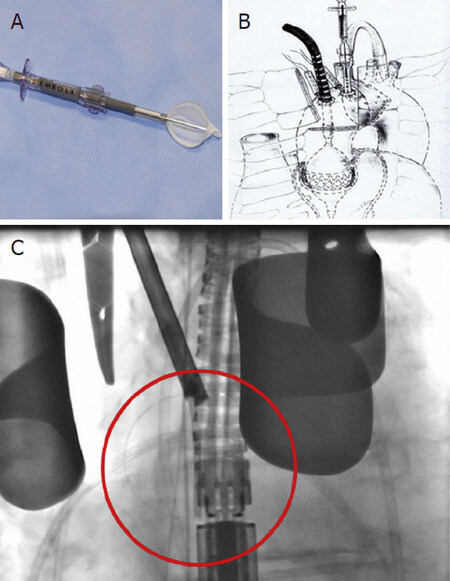
The Embrella Embolic Deflector (EED) system consists of an oval-shaped nitinol frame (length 59 mm, width 5-25 mm), covered with a porous polyurethane membrane (100 μm pore size). Its porous membrane allows blood flow to the brain while simultaneously deflecting embolic material [Figure 4]. The device is composed of 2 petals and a delivery cable in a 6F sheath system inserted via the right radial or brachial arteries. The two opposing petals are positioned along the aorta greater curvature, protecting the brachiocephalic and common carotid arteries from embolism[34,35].
Figure 4. Embrella device. Reproduced from Samim et al.[35]. A: the Embrella Embolic Deflector System; B: device positioned in the aortic arch; C: TAVI deployment
The EED System has been assessed in a limited clinical study in Europe and received CE Mark approval in May 2010. Two main RCT have studied the EED system. In these studies, Rodés-Cabau et al.[34] (PROTAVI-C Pilot) and Samim et al.[35] demonstrated the feasibility and safety of using the EED. However, the device failed to prevent cerebral microemboli or new transient ischemic lesions, as evaluated by Diffusion Weighted Imaging Magnetic Resonance Imaging (DW-MRI). In fact, the studies showed a higher number of brain lesions in the EED group compared to the control group, even though the device was associated with lower lesion volume[34,35]. The PROTAVI-C editorial comment also raises doubts about the real utility of the device[36].
ProtEmboR (Prtembis, GmbH, Germany)
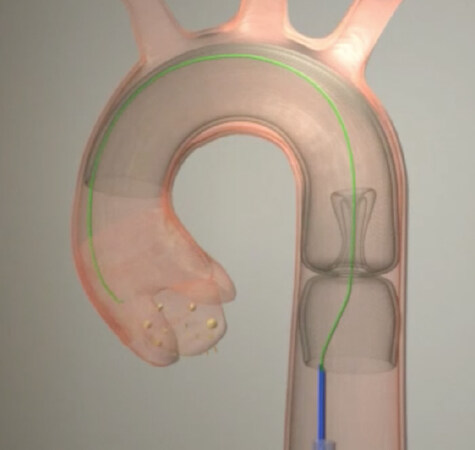
The ProtEmbo is an intra-aortic embolic protection filter device comprising a filter connected to a delivery unit enabling delivery of the unexpanded device with a 6F sheath via the left radial artery [Figure 5]. The device is delivered in the aortic arch, protecting its three major vessels (i.e., brachiocephalic trunk, left common carotid artery, and left subclavian artery). The filter consists of a porous polymeric material with 60 μm pores. The PROTEMBO SF Trial is the first randomized clinical trial to test the ProtEmbo device clinically. It has already completed the recruitment phase, but the results have not been published yet.
Emblok (Innovative Cardiovascular Solutions, Grand Rapids, MI, USA)
The Emblok embolic protection system is an 11F sheath device containing a 4F pigtail, delivered via femoral artery access in the aortic arch [Figure 6]. The device covers all three aortic arch vessels, and the filter consists of 125 μm of polyurethane.
The first trial testing the device was published on JACC, in 2020, by Latib et al.[37]. This prospective, nonrandomized, multicenter study had no control group. Nevertheless, it demonstrated that the use of this device appears to be feasible and safe. It was successfully placed and retrieved in all twenty cases, and no neurological events were observed.
Embol-X (Edwards Lifesciences, Irvine, CA, USA) - Transaortic
The Embol-X device was first developed to be used during open-heart surgery at the aortic cannulation site [Figure 7]. A randomized clinical trial tested its effectiveness in TAVI by a transaortic approach. In this trial, the device was shown to be safe and effective in reducing the incidence and the volume of new cerebral lesions. The device is placed inside the aorta and is available in 5 sizes covering an aortic diameter of 22 to 40 mm. It is delivered by a 17F sheath[38].
Figure 7. Transaortic Emblo-X device. Reproduced from Wendt et al.[38]. A: EMBOL-X system; B: transaortic TAVI; C: EMBOL-X intraprocedural control
Emboliner Embolic Protection Catheter (Emboline)TM
The Emboliner Cerebral Protection Catheter is the first device designed to prevent both cerebral and body embolism [Figure 8]. It is delivered through a transfemoral 9F sheath, the same sheath used for the 6F pigtail. Therefore, no additional access is required. Its pore size is 150 μm. The SafePass 2 trial is the first trial with the Emboliner device; it has completed enrollment but has not been published yet. However, the device seems to be safe and effective with little adverse events related to it, capturing up to five times more debris than Sentinel, according to informal data.
Cost-effectiveness analysis
There is no published cost-effectiveness analysis defining the real role of routine cerebral embolic protection device use during TAVI procedures. Therefore, the benefit of preventing a stroke should be balanced against the device costs, taking into consideration that strokes have an unpredictable, but often devastating impact, not only in terms of mortality but also in terms of sequelae (50% of patients develop permanent disability, more than 50% are unable to return to work, and more than 30% end up with serious financial problems). In this setting, Shiyovich et al.[39] estimated that the cost added by a moderate disability due to a neurologic event is around $25,000, followed by a subsequent annual cost increase of up to $60.000. Hence, as the device cost (Sentinel CPS) is approximately $2,800, and the CEPD number needed to treat is around 20, CEPD cost-effectiveness is suggested.
Discussion
To critically evaluate the CEPD trials presented above, some critical points should be taken into account. First, the studies showed important discrepancy between imaging and clinical outcomes since the observed reduction in new cerebral lesions number and volume did not reflect the expected benefit in hard outcomes. Trying to explain this discrepancy, it has been hypothesized that the lack of validated models to assess neurocognitive function in TAVI patients, the certain degree of pre-procedural cognitive dysfunction in some patients, and the high prevalence of inter and intra-observer variability for neurological tests, could blunt the real CEPD benefit[15]. Second, stroke incidence varies according to the study type, being significantly higher when the results are adjudicated based on formal neurologist clinical assessment (up to 10%) than when they are adjudicated by non-neurologists (2%-6%)[24-26]. Third, CEPD randomized trials have not been designed or powered to demonstrate an unequivocal impact on hard clinical endpoints. These observations make the search for preventive strategies even more relevant, especially in younger patients with longer life expectancy.
Regarding the best procedure strategy, we believe that it is still too early to affirm that CEPD should be universally used or that there is a specific patient population in which protected TAVI is more cost-effective. During the TAVI decision-making process, several factors should be balanced, such as age, the amount of leaflet and/or left ventricular outflow tract calcification, and the presence of aortic plaques or atrial fibrillation[26]. Therefore, from our perspective and considering the available evidence discussed above, two strategies could be possible:
Tailored preventive strategy: If TAVI is performed in a center with limited CEPD availability, one possible strategy could be to limit its use to high-risk scenarios based on preoperative risk factors (e.g., age, previous atrial fibrillation, history of cerebrovascular events, renal failure, concomitant coronary artery disease), transoperative risk factors (e.g., increased catheter and guidewire manipulation, extremely severe aortic stenosis, complex valve-in-valve procedures, multiple valve repositioning maneuvers, need for pre- and post-dilatation), and highly calcified anatomies (e.g., extensive atherosclerosis, complex aortic atheroma, bicuspid aortic valve, severe left ventricular outflow tract calcification).
Routine preventive strategy: If TAVI is performed in a center without CEPD use restrictions, one possible approach could be to offer it routinely as long as there is adequate anatomy, heart team indication, and patient concordance. This approach is based on the fact that captured debris are presented in almost all patients[
40 ], regardless of preoperative risk factors or type of device used.
Conclusion
This review article discusses the pros and cons of cerebral embolic protection use during TAVI procedures. Despite CEPD’s high cost, recent evidence, especially with the Sentinel system, has suggested that cerebral protection employment may lower stroke and even mortality rates. Ongoing and upcoming trials will help to fill some of the current evidence gaps related to CEPD use during TAVI.
Declarations
Authors’ contributionsMade substantial contributions to conception and design and review of this manuscript: Saadi EK, Saadi RP, Tagliari AP, Taramasso M
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestDr. Saadi EK is a consultant and Proctor for Medtronic, Abbott and Edwards and received speaker honoraria from Edwards and Medtronic. Dr. Tagliari AP has received a Research Grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (Capes) - Finance Code 001. Dr. Taramasso M is a consultant for Abbott Vascular, Boston Scientific, 4TECH, and CoreMedic; he has received speaker honoraria or Consultant fees from Edwards Lifesciences, CoreMedic, SwissVortex and Mitraltech.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
1. Velazquez EJ, Lee KL, Jones RH, et al; STICHES Investigators. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511-20.
2. Barker CM, Reardon MJ. The CoreValve US pivotal trial. Semin Thorac Cardiovasc Surg 2014;26:179-86.
3. Kolte D, Vlahakes GJ, Palacios IF, et al. Transcatheter versus surgical aortic valve replacement in low-risk patients. J Am Coll Cardiol 2019;74:1532-40.
4. Leon MB, Smith CR, Mack MJ, et al; PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20.
5. Mack MJ, Leon MB, Thourani VH, et al; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-705.
6. Popma JJ, Deeb GM, Yakubov SJ, et al; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706-15.
7. Reardon MJ, Van Mieghem NM, Popma JJ, et al; SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321-31.
8. Teitelbaum M, Kotronias RA, Sposato LA, Bagur R. Cerebral embolic protection in TAVI: friend or foe. Interv Cardiol 2019;14:22-5.
9. Kroon H, von der Thusen JH, Ziviello F, et al. Heterogeneity of debris captured by cerebral embolic protection filters during TAVI. EuroIntervention 2020:EIJ-D-20-00744.
10. Mastoris I, Schoos MM, Dangas GD, Mehran R. Stroke after transcatheter aortic valve replacement: incidence, risk factors, prognosis, and preventive strategies. Clin Cardiol 2014;37:756-64.
11. Eggebrecht H, Schmermund A, Voigtländer T, Kahlert P, Erbel R, Mehta RH. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 2012;8:129-38.
12. Nombela-Franco L, Webb JG, de Jaegere PP, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 2012;126:3041-53.
13. Muralidharan A, Thiagarajan K, Van Ham R, et al. Meta-analysis of perioperative stroke and mortality in transcatheter aortic valve implantation. Am J Cardiol 2016;118:1031-45.
14. Coylewright M, Palmer R, O’Neill ES, Robb JF, Fried TR. Patient-defined goals for the treatment of severe aortic stenosis: a qualitative analysis. Health Expect 2016;19:1036-43.
15. Armijo G, Nombela-Franco L, Tirado-Conte G. Cerebrovascular events after transcatheter aortic valve implantation. Front Cardiovasc Med 2018;5:104.
16. Seeger J, Gonska B, Otto M, Rottbauer W, Wöhrle J. Cerebral embolic protection during transcatheter aortic valve replacement significantly reduces death and stroke compared with unprotected procedures. JACC Cardiovasc Interv 2017;10:2297-303.
17. Giustino G, Mehran R, Veltkamp R, Faggioni M, Baber U, Dangas GD. Neurological outcomes with embolic protection devices in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2016;9:2124-33.
18. Kroon HG, van der Werf HW, Hoeks SE, et al. Early clinical impact of cerebral embolic protection in patients undergoing transcatheter aortic valve replacement: a two-center registry in the Netherlands. Circ Cardiovasc Interv 2019;12:1-8.
19. Nombela-Franco L, Armijo G, Tirado-Conte G. Cerebral embolic protection devices during transcatheter aortic valve implantation: clinical versus silent embolism. J Thorac Dis 2018;10:S3604-13.
20. Cubero-Gallego H, Pascual I, Rozado J, et al. Cerebral protection devices for transcatheter aortic valve replacement. Ann Transl Med 2019;7:584.
21. Jobanputra Y, Jones BM, Mohananey D, Fatima B, Kandregula K, Kapadia SR. Cerebral protection devices for transcatheter aortic valve replacement. Expert Rev Med Devices 2017;14:529-43.
22. Van Mieghem NM, van Gils L, Ahmad H, et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: the randomised MISTRAL-C trial. EuroIntervention 2016;12:499-507.
23. Ndunda PM, Vindhyal MR, Muutu TM, Fanari Z. Clinical outcomes of sentinel cerebral protection system use during transcatheter aortic valve replacement: a systematic review and meta-analysis. Cardiovasc Revasc Med 2020;21:717-22.
24. Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016;316:592-601.
25. Kapadia SR, Kodali S, Makkar R, et al; SENTINEL Trial Investigators. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol 2017;69:367-77.
26. Seeger J, Kapadia SR, Kodali S, et al. Rate of peri-procedural stroke observed with cerebral embolic protection during transcatheter aortic valve replacement: a patient-level propensity-matched analysis. Eur Heart J 2019;40:1334-40.
27. Cohen DJ. Cerebral embolic protection and TAVR outcomes: results from the TVT Registry. Presented at: TCT 2020. October 16, 2020. Available from: https://www.tctmd.com/slide/cerebral-embolic-protection-and-tavr-outcomes-results-tvt-registry. [Last accessed on 1 Dec 2020].
28. Megaly M, Sorajja P, Cavalcante JL, et al. Ischemic stroke with cerebral protection system during transcatheter aortic valve replacement. JACC Cardiovasc Interv 2020;13:2149-55.
29. Gasior T, Mangner N, Bijoch J, Wojakowski W. Cerebral embolic protection systems for transcatheter aortic valve replacement. J Interv Cardiol 2018;31:891-8.
30. Baumbach A, Mullen M, Brickman AM, et al. Safety and performance of a novel embolic deflection device in patients undergoing transcatheter aortic valve replacement: results from the DEFLECT I study. EuroIntervention 2015;11:75-84.
31. Lansky AJ, Schofer J, Tchetche D, et al. A prospective randomized evaluation of the TriGuard™ HDH embolic DEFLECTion device during transcatheter aortic valve implantation: results from the DEFLECT III trial. Eur Heart J 2015;36:2070-8.
32. Simsek C, Schölzel BE, den Heijer P, et al. The rationale of using cerebral embolic protection devices during transcatheter aortic valve implantation. Neth Heart J 2020;28:249-52.
33. Moses JW. A randomized evaluation of the TriGUARD3 cerebral embolic protection device to reduce the impact of cerebral embolic lesions after transcatheter aortic valve implantation: the REFLECT II trial. Presented at: TCT 2020. October 15, 2020. Available from: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2020/10/14/16/42/REFLECT-II. [Last accessed on 1 Dec 2020].
34. Rodés-Cabau J, Kahlert P, Neumann FJ, et al. Feasibility and exploratory efficacy evaluation of the Embrella Embolic Deflector system for the prevention of cerebral emboli in patients undergoing transcatheter aortic valve replacement: the PROTAVI-C pilot study. JACC Cardiovasc Interv 2014;7:1146-55.
35. Samim M, Agostoni P, Hendrikse J, et al. Embrella embolic deflection device for cerebral protection during transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2015;149:799-805.e1-2.
36. Van Mieghem NM, van der Lugt A. How embolism proof is the Embrella Embolic Deflector system? JACC Cardiovasc Interv 2014;7:1156-8.
37. Latib A, Mangieri A, Vezzulli P, et al. First-in-man study evaluating the emblok embolic protection system during transcatheter aortic valve replacement. JACC Cardiovasc Interv 2020;13:860-8.
38. Wendt D, Kleinbongard P, Knipp S, et al. Intraaortic protection from embolization in patients undergoing transaortic transcatheter aortic valve implantation. Ann Thorac Surg 2015;100:686-91.
39. Shiyovich A, Kornowski R. The use of embolic protection devices during transcatheter aortic valve implantation. Isr Med Assoc J 2019;21:615-9.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Saadi EK, Saadi RP, Tagliari AP, Taramasso M. Routine use of cerebral protection devices during transcatheter aortic valve implantation: what does the evidence say?. Vessel Plus 2020;4:41. http://dx.doi.org/10.20517/2574-1209.2020.54
AMA Style
Saadi EK, Saadi RP, Tagliari AP, Taramasso M. Routine use of cerebral protection devices during transcatheter aortic valve implantation: what does the evidence say?. Vessel Plus. 2020; 4: 41. http://dx.doi.org/10.20517/2574-1209.2020.54
Chicago/Turabian Style
Saadi, Eduardo Keller, Rodrigo Petersen Saadi, Ana Paula Tagliari, Maurizio Taramasso. 2020. "Routine use of cerebral protection devices during transcatheter aortic valve implantation: what does the evidence say?" Vessel Plus. 4: 41. http://dx.doi.org/10.20517/2574-1209.2020.54
ACS Style
Saadi, EK.; Saadi RP.; Tagliari AP.; Taramasso M. Routine use of cerebral protection devices during transcatheter aortic valve implantation: what does the evidence say?. Vessel Plus. 2020, 4, 41. http://dx.doi.org/10.20517/2574-1209.2020.54
About This Article
Copyright
Data & Comments
Data
 Cite This Article 19 clicks
Cite This Article 19 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.