Percutaneous mitral valve repair with the MitraClip in patients with handgrip exercise-induced dynamic mitral regurgitation
Abstract
Aim: To investigate whether patients with symptomatic heart failure and exercise-induced dynamic severe mitral regurgitation (MR) benefit from percutaneous mitral valve repair (PMVR).
Methods: We included patients who underwent PMVR with the MitraClip system in an all-comers observational study. Handgrip echocardiography was performed in patients with a discrepancy between symptoms and echocardiographic findings at rest, giving rise to the suspicion of an exercise-induced increase in MR severity. The primary endpoint of the study was a composite of all-cause mortality or admission for heart failure at 1-year follow-up. The secondary endpoint was the reduction in NYHA functional class.
Results: Two hundred twenty-one patients who underwent MitraClip implantation were included. Ninety-three patients with moderate to severe MR at rest received handgrip echocardiography prior to PMVR. The remaining 128 patients presented with severe MR at rest, making exercise echocardiography unnecessary. Handgrip exercise led to an increase in MR severity in 81% of patients with moderate MR at rest, irrespective of the subtype of MR. Following PMVR, patients with dynamic severe MR experienced comparable clinical improvement as patients with severe MR already at rest: During 1-year follow-up, 37 patients died, and 71 patients were re-admitted to the hospital because of heart failure. In this regard, 13 patients (30%) with dynamic severe MR experienced the combined endpoint, while 72 patients (43%) with severe MR at rest did as well (P = 0.121). Moreover, the majority of patients with dynamic severe MR similar to patients with severe MR at rest experienced clinical improvement from NYHA class III/IV to I/II (59% vs. 56%; P = 0.566).
Conclusion: The data presented provide evidence of a clinical benefit from PMVR using MitraClip in patients with moderate MR at rest who display exercise-induced increases in MR severity during handgrip exercise.
Keywords
Introduction
In recent years, cardiologists have increasingly recognized the dynamic nature of mitral regurgitation (MR)[1]. In degenerative MR (DMR) about one-third of patients with moderate to severe MR display notable exercise-induced increases in MR severity [increase in effective regurgitant orifice area (EROA) > 10 mm2 and regurgitation volume (RVol) >15 mL][2]. Owing to these findings, there is a recommendation for exercise echocardiography during the diagnostic work-up of symptomatic patients with DMR in whom there is a discrepancy between symptoms and the severity of MR at rest[3,4]. In functional MR (FMR) exercise-induced changes in MR severity are also common and may provide prognostic information on the clinical course of heart failure[5-7]. European Society of Cardiology (ESC) guidelines emphasize the role of exercise echocardiography in unmasking significant dynamic MR in patients with left ventricular systolic dysfunction, since resting MR severity may not correlate with the potentially clinical significant increase in MR during exercise[4]. Percutaneous mitral valve repair (PMVR) with the MitraClip system has emerged as a treatment option for patients with DMR and FMR, who are inoperable or have a high surgical risk. However, current recommendations do not cover dynamic MR since data on the safety and efficacy of PMVR are lacking in these patients. Likewise, there are only scarce data on exercise echocardiography in patients undergoing PMVR, of whom the majority may be too frail to undergo traditional bicycle exercise testing.
In the current study, we report our experience of PMVR using the MitraClip in patients with moderate MR at rest and dynamic severe MR during handgrip exercise, compared to patients with severe MR already at rest.
Methods
Study design
We included patients who underwent PMVR with the MitraClip system at our institution from 2012 to 2016 in an all-comers observational study. Patients with moderate to severe MR at rest received handgrip echocardiography prior to MitraClip implantation. Handgrip echocardiography was performed in patients with a discrepancy between symptoms and echocardiographic findings at rest, giving rise to the suspicion of an exercise-induced increase in MR severity. Our interdisciplinary heart team classified all patients as inoperable or at high-risk for surgery. The study was performed in accordance with the Declaration of Helsinki and was part of a registry, which was approved by the local ethics committee of the Heinrich-Heine University and registered at www.clinicaltrials.gov (NCT02033811).
Follow-up
Patients were routinely followed by referring cardiologists and scheduled for a single outpatient visit 12 months after MitraClip implantation in our specialty clinic for structural heart disease. The clinical course of patients in whom this single follow-up examination in our department was deemed impossible was monitored by telephone interview with referring cardiologists and the patients’ primary physicians or the patients themselves. The primary endpoint of the study included a composite of all-cause mortality or hospitalization for heart failure during 12 months follow-up. Hospitalization for heart failure was defined by clinical symptoms suggestive of heart failure (e.g., worsening of dyspnea, edema and fatigue) and the need for i.v. diuretics during re-hospitalization. Secondary endpoint was reduction in New York Heart Association (NYHA) functional class at 12 months following PMVR compared to baseline.
Echocardiographic evaluation
Echocardiographic examinations were performed using a GE Vivid S6/E9 or a Philips iE33. All echocardiographic data were stored on a workstation for offline analysis (Xcelera Cardiology Information Management, Philips). Left ventricular volumes and ejection fraction were calculated using the Simpson biplane method. Systolic pulmonary artery pressure (SPAP) was estimated from the regurgitant jet of tricuspid regurgitation with peak systolic trans-tricuspid pressure gradient. Assessment of MR was performed according to current guidelines[4]. MR severity was assessed by an integrative approach using semi-quantitative [vena contracta (VC)] and quantitative (EROA, RVol) parameters. The severity of MR was graded mild (1+), moderate (2+) and severe (3+). In patients with secondary MR, the cutoff values for severe MR were: EROA 20 mm2 and RVol 30 mL, according to current guidelines[4]. The radius of the maximal proximal iso-velocity surface area (PISA) was measured using several frames. The largest radius was selected for analysis. RVol and EROA were calculated with standard formulae as previously described. For semi-quantitative assessment, VC width was assessed from the apical four-chamber view. The largest VC diameter was measured for three cardiac cycles and averaged. Patients presenting with moderate MR at rest and severe MR during handgrip exercise were assigned “dynamic severe MR”.
Handgrip exercise
Following comprehensive echocardiographic examination at rest, handgrip exercise was performed according to a standardized protocol with a handgrip dynamometer (Jamar® Hydraulic Hand Dynamometer, Sammons Preston Inc.) while the patient lay on his/her left side. After initial recording of blood pressure and heart rate at rest, the patient was asked to squeeze the dynamometer with maximum effort for a short period only. The handgrip exercise was then carried out at half-maximum force for three minutes, while echocardiographic data were obtained focusing on MR and SPAP. Blood pressure and heart rate were recorded every minute. MR was quantified as indicated above, including measurement of PISA, VC, EROA and RVol. In case of non-reproducible results according to the PISA method (e.g., due to eccentric jets in 8 of 93 patients), VC width was used for assessment of MR severity. Medical therapy (including ß-blockers) remained unchanged for the exercise test.
Statistical analysis
All analyses were performed using Sigma Plot software (Version 11.0, Systat Software Ltd). Results are expressed as mean ± standard deviation or percentage unless otherwise specified. Normality distribution was assessed with the Kolmogorov-Smirnov test. A two-tailed paired t-test was used to compare differences between two groups. Fisher’s exact test was used to investigate the significance of the association between two kinds of classification. Differences between three groups were assessed by one-way ANOVA with post-hoc Tukey correction for multiple comparisons. The Kaplan-Meier method and the log-rank test were used for presenting the event-free rate. For all analyses, a P-value of < 0.05 was considered statistically significant
Results
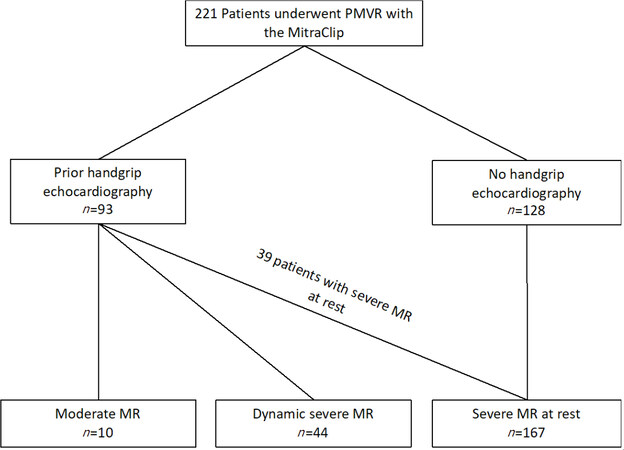
We included 221 patients who underwent PMVR with the MitraClip system. Ninety-three patients with moderate to severe MR at rest received handgrip echocardiography prior to MitraClip implantation [Figure 1]. The remaining 128 patients presented with clinically relevant severe MR at rest, making exercise echocardiography unnecessary.
Figure 1. Consort diagram. Two hundred twenty-one patients with complete echocardiographic and follow-up data were included in retrospect. In 93 patients, handgrip echocardiography was performed prior to the procedure. Of those 93 patients, 44 patients presented with moderate MR at rest and dynamic severe MR during handgrip. Thirty-nine patients had severe MR already at rest. However, 10 patients showed moderate MR at rest and during exercise, but nevertheless underwent MitraClip implantation because of persistent severe symptoms. In addition, 128 patients with severe MR at rest were included, who did not undergo handgrip echocardiography. MR: mitral regurgitation
Demographic and clinical data of the cohort are summarized in Table 1. Mean age was 75 ± 10 years, 36% were female. One hundred ninety-six patients (89%) presented with NYHA functional class III or IV. Eighty patients (36%) had DMR, while 141 patients (64%) presented with FMR [Table 2]. One hundred sixty-seven patients (76%) fulfilled criteria for severe MR at rest according to current recommendations [Figure 1][4]. The remaining patients presented with moderate MR at rest (n = 54, 24%). As expected, patients with severe MR at rest displayed more pronounced elevations in NT-proBNP (P = 0.015) and had a higher logistic EuroSCORE (P = 0.037) compared to patients with moderate MR and dynamic severe MR [Table 1]. Regarding echocardiographic parameters, LA area was larger in patients with severe MR at rest compared to patients with dynamic MR [Table 2]. In addition, LVEF tended to be lower in patients with severe MR at rest compared to patients with dynamic MR (46% ± 12% vs. 42% ± 13%; P = 0.053). Furthermore, SPAP was numerically increased in patients with severe MR at rest compared to patients with dynamic MR, however, without reaching statistical significance (47 ± 15 vs. 42 ± 12 mmHg; P = 0.345).
Baseline patient characteristics
| All patients n = 221 | Moderate MR n = 10 | Dynamic severe MR n = 44 | Severe MR at rest n = 167 | P-value | |
|---|---|---|---|---|---|
| Age (years) | 75 ± 10 | 77 ± 8 | 77 ± 9 | 75 ± 11 | 0.696 |
| Gender, female, n (%) | 80 (36%) | 6 (60%) | 21 (48%) | 53 (32%) | 0.040 |
| Hypertension, n (%) | 183 (83%) | 9 (90%) | 35 (80%) | 142 (85%) | 0.594 |
| Diabetes, n (%) | 57 (26%) | 4 (40%) | 12 (27%) | 41 (25%) | 0.541 |
| Previous cardiac history | |||||
| ICM, n (%) | 151 (68%) | 9 (90%) | 28 (64%) | 114 (68%) | 0.290 |
| DCM, n (%) | 38 (17%) | 1 (10%) | 11 (25%) | 26 (16%) | 0.107 |
| Previous CABG, n (%) | 73 (33%) | 5 (50%) | 12 (27%) | 56 (34%) | 0.403 |
| Previous VS, n (%) | 40 (18%) | 4 (40%) | 5 (11%) | 31 (19%) | 0.117 |
| Atrial fibrillation, n (%) | 121 (55%) | 4 (40%) | 27 (61%) | 90 (54%) | 0.430 |
| Logistic EuroSCORE (%) | 22 ± 15 | 17 ± 7 | 18 ± 12 | 24 ± 16* | 0.037 |
| NYHA functional class | 0.425 | ||||
| NYHA II, n (%) | 24 (11%) | 1 (10%) | 6 (14%) | 17 (10%) | |
| NYHA III, n (%) | 156 (71%) | 7 (70%) | 33 (75%) | 116 (69%) | |
| NYHA IV, n (%) | 40 (18%) | 2 (20%) | 4 (9%) | 34 (20%) | |
| Labaratory assessment | |||||
| Hemoglobin (mg/dL) | 12.1 ± 1.9 | 11.2 ± 2.4 | 12.4 ± 1.8 | 12.1 ± 1.9 | 0.185 |
| eGFR (mL/min/1.73 m2) | 51 ± 23 | 50 ± 17 | 56 ± 17 | 51 ± 24 | 0.853 |
| NT-proBNP (pg/mL) | 4499 ± 6499 | 2077 ± 1917 | 2161 ± 1555 | 5158 ± 7048* | 0.015 |
Echocardiographic parameters at rest
| All patients n = 221 | Moderate MR n = 10 | Dynamic severe MR n = 44 | Severe MR at rest n = 167 | P-value | |
|---|---|---|---|---|---|
| DMR (%) | 80 (36%) | 5 (50%) | 18 (41%) | 57 (34%) | 0.478 |
| FMR (%) | 141 (64%) | 5 (50%) | 26 (59%) | 110 (66%) | 0.478 |
| LVEDD (mm) | 56 ± 11 | 55 ± 8 | 55 ± 10 | 57 ± 10 | 0.106 |
| LA area (cm2) | 28 ± 9 | 26 ± 6 | 25 ± 7 | 29 ± 10* | 0.024 |
| LVEF (%) | 43 ± 13 | 48 ± 13 | 46 ± 12 | 42 ± 13 | 0.053 |
| RV diameter (mm) | 30 ± 7 | 29 ± 3 | 29 ± 8 | 31 ± 7 | 0.548 |
| TAPSE (mm) | 18 ± 4 | 19 ± 4 | 19 ± 4 | 18 ± 4 | 0.888 |
| VC width (mm) | 6.4 ± 1.8 | 5.3 ± 1.2 | 5.3 ± 1.0 | 6.7 ± 1.9* | 0.002 |
| PISA radius (mm) | 7.4 ± 3.1 | 5.9 ± 0.5 | 6.4 ± 1.0 | 7.8 ± 1.8#,* | < 0.001 |
| RVol (mL) | 43 ± 24 | 25 ± 5 | 30 ± 11 | 48 ± 20#,* | 0.001 |
| EROA (mm2) | 28 ± 12 | 19 ± 7 | 20 ± 8 | 30 ± 17#,* | 0.007 |
| MVPG (mmHg) | 2.2 ± 1.0 | 1.5 ± 0.6 | 1.9 ± 0.8 | 2.3 ± 1.1 | 0.041 |
| Moderate/severe TR, n (%) | 76 (34%) | 5 (50%) | 16 (36%) | 55 (31%) | 0.515 |
| SPAP (mmHg) | 45 ± 14 | 50 ± 16 | 42 ± 12 | 47 ± 15 | 0.345 |
Handgrip exercise testing
Handgrip exercise resulted in a meaningful hemodynamic response. Heart rate increased from 67 ± 12 beats/min (bpm) at rest to 78 ± 14 bpm with handgrip exercise (P < 0.001). Systolic blood pressure increased from 123 ± 21 mmHg at rest to 138 ± 23 mmHg with exercise (P < 0.001), and diastolic blood pressure increased as well from 66 ± 14 mmHg to 73 ± 20 mmHg (P = 0.005). Rate pressure product showed an increase from 8186 ± 2074 to 10735 ± 3043 mmHg*bpm (P < 0.001) during handgrip exercise. No patient experienced chest pain, ischemic ECG changes or significant arrhythmias during exercise.
Exercise-induced changes of MR severity and SPAP
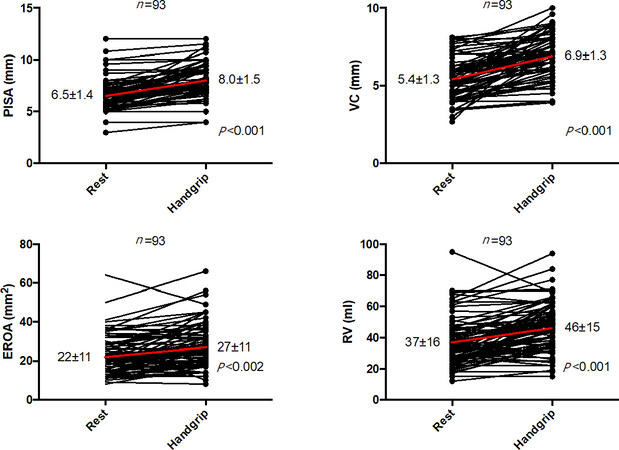
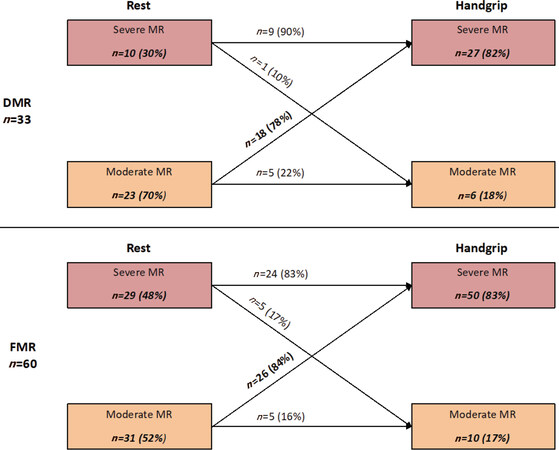
Handgrip exercise caused an increase in PISA radius, EROA and RVol (all P < 0.01) [Figure 2]. According to established parameters, 44 patients (81%) with moderate MR at rest showed dynamic severe MR during handgrip exercise. Stratified by MR etiology, 18 patients (78%) with moderate DMR at rest showed exercise-induced increase in MR severity (dynamic severe MR) [Figure 3]. Similarly, 26 patients (84%) with moderate FMR at rest revealed dynamic severe MR during handgrip exercise. SPAP estimated by peak tricuspid regurgitation jet velocity increased from 42 ± 12 mmHg to 50 ± 13 mmHg (P < 0.001). Thirty patients (32%) had pre-existing pulmonary hypertension (PH) at rest and two other patients exhibited PH (> 60 mmHg) during handgrip exercise.
Figure 2. Individual changes in echocardiographic parameters during handgrip exercise (n = 93). Exercise-induced changes in PISA, VC, EROA and RVol are shown with mean values (red). During handgrip exercise, we observed a significant increase in different echocardiographic parameters indicating dynamic MR. In case of non-reproducible results according to the PISA method (e.g., due to eccentric jets in 8 of 93 patients), VC width was used for assessment of MR severity. PISA: proximal iso-velocity surface area; EROA: effective orifice regurgitant area; MR: mitral regurgitation; RVol: regurgitation volume; VC: vena contracta
Figure 3. Exercise-induced changes in MR severity during handgrip exercise (n = 93). Eighteen patients (78%) with moderate DMR at rest and 26 patients (84%) with moderate FMR at rest revealed exercise-induced increase in echocardiographic parameters representing dynamic severe MR. Note that only patients who underwent PMVR were included and not those who were not selected for PMVR due to remaining mild or moderate MR during exercise, making a selection bias probable. However, 10 patients with moderate MR at rest and during exercise underwent MitraClip implantation due to persistent severe symptoms. MR: mitral regurgitation; DMR: degenerative mitral regurgitation; FMR: functional mitral regurgitation
Procedural results and overall clinical outcome
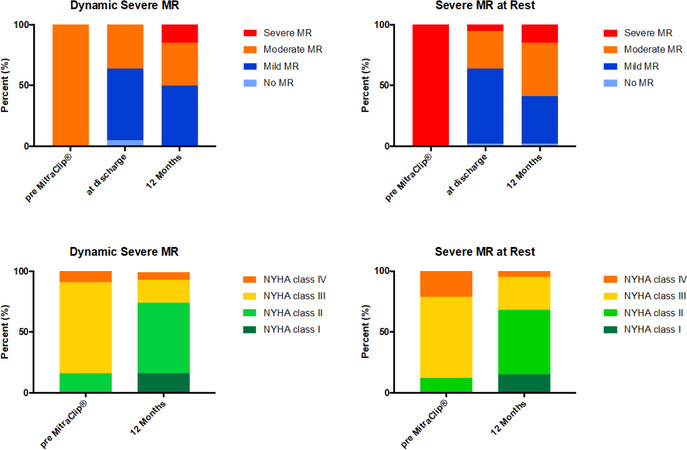
Acute procedural success, defined by a reduction to MR grade ≤ 2+ was achieved in 215 patients (97%). PMVR with the MitraClip system was equally effective in patients with and without dynamic MR, as indicated by a reduction of MR grade ≤ 1+ at discharge in 61% of patients with dynamic severe MR and 59% of patients with severe MR at rest (P > 0.999), respectively [Figure 4].
Figure 4. Change in MR severity at rest and NYHA functional class pre-MitraClip implantation, at discharge and at 1-year follow-up in patients with dynamic severe MR (n = 44) and severe MR at rest (n = 167). MR severity at rest and NYHA functional class improved following MitraClip independent of the presence of dynamic MR. MR: mitral regurgitation; NYHA: New York Heart Association; PMVR: percutaneous mitral valve repair
Follow-up was complete in all patients (100%). During 12 ± 4 months follow-up, 37 patients (17%) died and 71 patients (32%) were re-admitted to the hospital because of heart failure symptoms. In the whole cohort, 92 patients (42%) experienced at least one event. Furthermore, 70% of patients belonged to NYHA functional class I or II (in contrast to 11% at baseline) (data not shown). In 93% of the surviving patients (n = 174), echocardiography after 12 ± 4 months was performed. MR grade improved compared to baseline MR grade ≤ 2+ in 85% of surviving patients.
Comparable clinical benefit in patients with dynamic severe MR and severe MR at rest
Effective reduction of MR following MitraClip implantation was associated with comparable clinical improvements in patients with dynamic severe MR and patients with severe MR at rest. At 12 ± 4 months, in patients with exercise-induced dynamic severe MR, all-cause mortality was half of the patients with severe MR at rest (9% vs. 17%), however, without reaching statistical significance (P = 0.244) [Table 3]. Similarly, the rate of heart failure rehospitalizations was numerically lower in patients with dynamic MR compared with those with severe MR at rest (25% vs. 33%), but also not of statistical significance (P = 0.364) [Table 3]. Thus, the combined endpoint reached was equally frequent in the two patient cohorts (30% vs. 43%; P = 0.121). Respective Kaplan-Meier survival curves are given in Supplementary Figure 1. Four out of 10 patients with moderate MR at rest and during exercise died during follow-up [Table 3]. There was no death and no admission for heart failure in patients with a decrease in MR severity during exercise (severe MR at rest and moderate MR during exercise) (data not shown). Seventy-four per cent of patients with dynamic severe MR and 68% of patients with severe MR at rest belonged to NYHA class I or II [Figure 4]. In this regard, 59% of patients with dynamic severe MR and 56% of patients with severe MR at rest experienced a clinical improvement from NYHA class III/IV to I/II (P > 0.999) during one-year follow-up. Eighty-five per cent of patients with dynamic severe MR showed MR grade ≤ 2+, and 85% of patients with severe MR at rest did as well (P > 0.999) [Figure 4].
Distribution of post-procedural outcome after 12 ± 4 months according to the grade of MR severity and to the presence of dynamic MR at baseline
| All patients n = 221 | Moderate MR n = 10 | Dynamic severe MR n = 44 | Severe MR at rest n = 167 | P-value | |
|---|---|---|---|---|---|
| All-cause mortality, n (%) | 37(17) | 4(40) | 4(9) | 29(17) | 0.244 |
| Cardiac death, n (%) | 18(49) | 2(50) | 2(50) | 14(48) | 0.532 |
| Non-cardiac death, n (%) | 10(27) | 1(25) | 1(25) | 8(28) | 0.689 |
| Unknown, n (%) | 9(24) | 1(25) | 1(25) | 7(24) | 0.999 |
| HF-admission, n (%) | 71(32) | 5(50) | 11(25) | 55(33) | 0.364 |
| Mortality or HF-admission, n (%) | 92(42) | 7(70) | 13(30) | 72(43) | 0.121 |
Discussion
In the current study, we assessed the therapeutic benefit from PMVR with the MitraClip system in patients with dynamic severe MR. We demonstrated that the symptomatic benefit following PMVR in patients with dynamic severe MR, assessed during handgrip exercise, was similar compared to those patients presenting with severe MR already at rest, irrespective of the etiology of MR (degenerative and functional MR).
Effects of MitraClip implantation on dynamic MR
At one-year follow-up, MitraClip implantation was associated with reduction in symptoms as assessed by NYHA functional class and improvement in MR severity as assessed by echocardiography. Our findings are in line with the results from the EVEREST-II trial, supporting MitraClip therapy mainly in primary MR[8], as well as with the results from real-world registries deciphering clinical improvement in patients with predominately secondary MR[9]. Notably, there was a similar clinical benefit (regarding all-cause mortality, hospitalization for heart failure and reduction of NYHA functional class) and no difference in reduction of MR severity in patients with dynamic severe MR compared to patients with severe MR already at rest.
Our findings here support the performance of exercise echocardiography in patients displaying moderate MR at rest but reporting high-grade MR from a previous echocardiography or presenting with symptoms suggestive of dynamic MR. Recently, Van de Heyning et al.[10] provided the first clinical evidence of hemodynamic improvements during exercise following PMVR in patients with secondary MR. They compared exercise echocardiography before and six months after MitraClip implantation. In 31 patients, a significant reduction of MR at peak exercise along with increased calculated cardiac output and decreased pulmonary arterial pressures (measured echocardiographically by trans-tricuspid pressure gradient) was documented. Here, we demonstrated in a larger, all-comers cohort that these findings go along with reduction in symptoms as assessed by NYHA functional class and an improvement in MR severity. The findings presented are of clinical relevance because MR resembles a dynamic entity that is sensitive to changes in preload, afterload and ventricular geometry as well[1]. Even in patients with only mild or moderate MR at rest, Lapu-Bula et al.[11] demonstrated a relevant negative impact on exercise-induced MR deterioration on exercise capacity. This might have been due to the combination of an inhibition of the expected increase in exercise forward stroke volume and a marked increase in pulmonary artery pressure[11]. Recently, Lancellotti et al.[12] provided clinical evidence to understand the unfavorable consequences of dynamic MR. They described that SPAP during exercise is associated with dynamic increase in EROA in patients with secondary MR and revealed a significant prognostic importance of exercise-induced PH, regarding the occurrence of cardiac death and cardiac events. The prognostic impact of dynamic MR has also been described by others[2,6,13]. In this regard, mortality in patients with deteriorating secondary MR during exercise managed with optimal medical therapy exhibited increased mortality in the range of patients with severe MR already at rest[6]. In the present study, we demonstrated a similar benefit in patients with dynamic severe MR and patients with severe MR at rest with regard to outcomes such as all-cause mortality, hospitalization for heart failure and symptomatic improvements. This may be explained by an effective reduction of dynamic MR by PMVR, as optimal reduction of MR seems to be of utmost importance for long-term clinical outcome[14]. Mechanistically, PMVR with MitraClip implantation effectively increases coaptation area, thus improving closing force efficiency[15] with an additional decrease in annular dimensions (antero-posterior)[16]. This may lead to reduction in MR severity not only at rest, but also during exercise as has been recently shown by Van de Heyning et al.[10] and is accompanied by relief from dyspnea.
The role of handgrip echocardiography prior to MitraClip implantation
Our study demonstrates that handgrip exercise serves as a valuable tool to unmask dynamic changes in MR. According to pre-defined parameters [Supplementary Figure 2], 15 patients (45%) with DMR and 24 patients (40%) with FMR revealed a marked increase in MR severity (EROA > 10 mm2, RVol > 15 mL) during handgrip exercise, irrespective of MR severity at rest. These data foster previous results from studies reporting dynamic MR during exercise in one-third of patients with DMR[17] and in 30%-50% of cases with FMR, both ischemic and non-ischemic etiology[18-21]. Increasing MR severity during handgrip exercise is exaggerated by the hemodynamic response[22], which mainly imposes pressure load on the left ventricle due to an increase in systemic vascular resistance, whereas dynamic exercise predominately results in volume overload[23]. We decided to perform handgrip exercise as we included a frail patients cohort (logistic EuroSCORE 22% ± 15%) of whom the majority might have been too unfit to complete bicycle exercise. The increase in blood pressure during isometric exercise may lead to a mismatch between increasing mitral closing force and increased mitral tethering resulting from the impact of the rise in afterload on left ventricular geometry[24]. Additional mechanisms such as left ventricular dyssynchrony, changed sphericity and papillary muscle dynamics during exercise, may enhance these forces.
Limitations
The present study was retrospective in design so that we did not include a control group with moderate MR at rest and exercise-induced severe MR that was managed conservatively with optimal medical therapy. However, this would be necessary to definitively conclude the clinical benefit of patients with moderate MR at rest and exercise-induced dynamic MR. Moreover, this study was conducted at a single center. Therefore, further validation in a larger prospective multicenter study will be necessary to confirm the present findings. However, this is the largest study including patients with dynamic MR undergoing PMVR, so far. Since exercise echocardiography was performed upon clinical suspicion of dynamic MR and not on regular basis in every patient with MR grade 2+, a selection bias towards patients with relatively advanced disease in MR grade 2+ seems probable. In addition, we only included patients who underwent PMVR and not those who were not selected for PMVR because of remaining mild or moderate MR during exercise. These questions need to be addressed in prospective analyses.
In conclusion, our data provide clinical evidence of symptomatic benefit from PMVR (MitraClip) in patients with dynamic severe MR detected by handgrip echocardiography. These patients equally benefit from MitraClip implantation as patients with severe MR at rest. Further studies comparing an interventional strategy (PMVR) vs. conservative management (optimal medical therapy) are necessary to further evaluate clinical benefit from PMVR in patients with dynamic MR. Handgrip echocardiography may be integrated in the diagnostic workup of symptomatic patients with moderate MR at rest with suspicion of dynamic MR based on discrepancy between symptoms and echocardiographic findings at rest.
Declarations
Authors’ contributions
Designed the study: Spieker M, Hellhammer K, Horn P, Westenfeld R
Responsible for data collection: Spieker M, Hellhammer K
Performed the statistical analysis: Spieker M, Westenfeld R
Involved in data interpretation, critically reviewed and revised the manuscript: Spieker M, Hellhammer K, Spießhoefer J, Zeus T, Horn P, Kelm M, Westenfeld R
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no no conflicts of interest.
Ethical approval and consent to participate
The study was part of a registry, which was approved by the ethics committee of the Heinrich-Heine University Duesseldorf and registered at www.clinicaltrials.gov (NCT02033811). The study was in accordance with the Declaration of Helsinki. All patients gave informed written consent.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
REFERENCES
2. Magne J, Lancellotti P, Piérard LA. Exercise-induced changes in degenerative mitral regurgitation. J Am Coll Cardiol 2010;56:300-9.
3. Bonow R, Carabello B, Erwin J, Guyton RA, O’Gara PT, et al. 2014 AHA/ACC guideline forthe Management of patients with valvular heart disease: executive summary. J Am Coll Cardiol 2014;63:1-169.
4. Baumgartner H, Falk V, Bax J, De Bonis M, Hamm CW, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91.
5. Bertrand PB, Schwammenthal E, Levine RA, Vandervoort PM. Exercise dynamics in secondary mitral regurgitation. Circulation 2017;135:297-314.
6. Lancellotti P, Gérard PL, Piérard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J 2005;26:1528-32.
7. Lancellotti P. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N Engl J Med 2004;351:1627-34.
8. Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) high risk study. J Am Coll Cardiol 2012;59:130-9.
9. Maisano F, Franzen O, Baldus S, Schäfer U, Hausleiter J, et al. Percutaneous mitral valve interventions in the real world. J Am Coll Cardiol 2013;62:1052-61.
10. Van De Heyning CM, Bertrand PB, Debonnaire P, De Maeyer C, Vandervoort PM, et al. Mechanism of symptomatic improvement after percutaneous therapy for secondary mitral regurgitation resting and exercise hemodynamics. J Am Coll Cardiol 2016;68:128-9.
11. Lapu-Bula R, Robert A, Van Craeynest D, D’Hondt AM, Gerber BL, et al. Contribution of exercise-induced mitral regurgitation to exercise stroke volume and exercise capacity in patients with left ventricular systolic dysfunction. Circulation 2002;106:1342-8.
12. Lancellotti P, Magne J, Dulgheru R, Ancion A. Clinical significance of exercise pulmonary hypertension in secondary mitral regurgitation. Am J Cardiol 2015;115:1454-61.
13. Lancellotti P, Troisfontaines P, Toussaint A, Pierard LA. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation 2003;108:1713-7.
14. Puls M, Lubos E, Boekstegers P, Von Bardeleben RS, Ouarrak T, et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J 2016;37:703-12.
15. Al Amri I, Debonnaire P, Witkowski T, Van Der Kley F, Palmen M, et al. Mitral valve geometry and hemodynamics after surgical mitral valve annuloplasty and implications for percutaneous treatment of patients with recurrent mitral regurgitation. Am J Cardiol 2013;112:714-9.
16. Schmidt FP, Von Bardeleben RS, Nikolai P, Jabs A, Wunderlich N, et al. Immediate effect of the MitraClip® procedure on mitral ring geometry in primary and secondary mitral regurgitation. Eur Heart J Cardiovasc Imaging 2013;14:851-7.
17. Magne J, Lancellotti P, Piérard LA. Exercise-induced changes in degenerative mitral regurgitation. J Am Coll Cardiol 2010;56:300-9.
18. Lebrun F, Lancellotti P, Piérard LA. Quantitation of functional mitral regurgitation during bicycle exercise in patients with heart failure. J Am Coll Cardiol 2001;38:1685-92.
19. Lancellotti P, Troisfontaines P, Toussaint AC, Pierard LA. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation 2003;108:1713-7.
20. Lancellotti P, Stainier P, Lebois F, Piérard LA. Effect of dynamic left ventricular dyssynchrony on dynamic mitral regurgitation in patients with heart failure due to coronary artery disease. Am J Cardiol 2005;98:1304-7.
21. Vecera J, Bartunek J, Vanderheyden M, Kotrc M, Kockova R, et al. Three-dimensional echocardiography-derived vena contracta area at rest and its increase during exercise predicts clinical outcome in mild-moderate functional mitral regurgitation. Circ J 2014;78:2741-9.
22. Crawford MH, White DH, Amon KW. Echocardiographic evaluation of left ventricular size and performance during handgrip and supine and upright bicycle exercise. Circulation 1979;59:1188-97.
23. Manou-Stathopoulou V, Goodwin CD, Patterson T, Redwood SR, Marber MS, et al. The effects of cold and exercise on the cardiovascular system. Heart 2015;101:808-20.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Spieker M, Hellhammer K, Spießhoefer J, Zeus T, Horn P, Kelm M, Westenfeld R. Percutaneous mitral valve repair with the MitraClip in patients with handgrip exercise-induced dynamic mitral regurgitation. Vessel Plus 2020;4:29. http://dx.doi.org/10.20517/2574-1209.2020.28
AMA Style
Spieker M, Hellhammer K, Spießhoefer J, Zeus T, Horn P, Kelm M, Westenfeld R. Percutaneous mitral valve repair with the MitraClip in patients with handgrip exercise-induced dynamic mitral regurgitation. Vessel Plus. 2020; 4: 29. http://dx.doi.org/10.20517/2574-1209.2020.28
Chicago/Turabian Style
Spieker, Maximilian, Katharina Hellhammer, Jens Spießhoefer, Tobias Zeus, Patrick Horn, Malte Kelm, Ralf Westenfeld. 2020. "Percutaneous mitral valve repair with the MitraClip in patients with handgrip exercise-induced dynamic mitral regurgitation" Vessel Plus. 4: 29. http://dx.doi.org/10.20517/2574-1209.2020.28
ACS Style
Spieker, M.; Hellhammer K.; Spießhoefer J.; Zeus T.; Horn P.; Kelm M.; Westenfeld R. Percutaneous mitral valve repair with the MitraClip in patients with handgrip exercise-induced dynamic mitral regurgitation. Vessel Plus. 2020, 4, 29. http://dx.doi.org/10.20517/2574-1209.2020.28
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 4 clicks
Cite This Article 4 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.