Characterizing the mechanical properties of the aortic wall
Abstract
Characterizing the physical properties of the aortic wall is essential to understanding the causes of cardiovascular diseases, such as aneurysms. Modelling compliant, anisotropic, multilayered tubes such as the aorta has proven to be a challenge. In vitro studies of the mechanical properties of arteries incorporate a variety of testing methods; however, the majority of these tests fail to replicate the complex, transmural loading conditions arising from pulsatile flow. These methods include typical tensile tests, both in uniaxial and biaxial set-ups, bulge inflation tests and extension-inflation tests. Bulge-inflation tests grant material information in response to biaxial loading but still do not mimic proper cylindrical loading conditions. Extension-inflation tests capture the cylindrical loading but have only been performed with static pressurization and with rigid boundary conditions in effect. This review aims to present the current state of the biomechanical characterization of arterial walls, particularly the aorta, through discussion of testing methods and their findings. We emphasize literature that focuses on prediction of aneurysm rupture risk. Moreover, overarching concepts such as histological effects, age dependent effects, segmental effects, hemodynamic effects, viscoelastic modelling and torsion will be briefly explored. An understanding of the current limitations of testing will hopefully lead to the development of more robust in vitro test methods that will further elucidate the relationship between changing vessel wall mechanics and cardiovascular disease.
Keywords
Introduction
Aortic aneurysm can be a life-threatening condition, representing a serious mortality risk of 80% if rupture occurs[1]. There is a significant decline in mortality risk if aneurysms are electively treated with aortic replacements when compared to non-elective replacements[2]. Therefore, early predictions of aneurysm rupture risk can be of great benefit to patients. Currently, an aortic aneurysm diameter greater than 5.5 cm is an indication for intervention. However, in patients with connective tissue disorders, where structural changes in the aortic wall are well defined, a much lower threshold of dilatation is warranted for intervention; in Marfan Syndrome, a diameter of greater than 5.0 cm is an indication for surgery with a lower threshold of 4.5 cm in the presence of further risk factors[3].
Aneurysm diameter and growth may not accurately predict the risk of aneurysm rupture[4] and might lead to undertreatment of those who have other contributing factors such as aortic stiffness or a connective tissue disorder. Alternatively, overtreatment is also a possibility: Trabelsi et al.[5] found that even at a diameter of 6 cm only 31% of patients with aneurysms developed complications. Moreover, in the general population, there is a large number of individuals with aortic diameters between 5.0 and 5.5 cm whose risk of complications are not well understood[6]. The Laplace Law is the theoretical basis of the diameter guidelines; however, this law is valid for simple spheres and uniform cylinders and might not adequately appreciate the complex geometrical nature of the native vessels[1].
The biomechanical analysis of diseased aortas could therefore represent an intriguing opportunity to further elucidate the mechanisms leading to rupture even in the absence of connective tissue disorder. It has long been recognized that a blood vessel cannot be considered as a passive conduit for blood flow. Rather, a blood vessel is a continuously adapting, dynamic element with the purpose of maintaining optimal function in response to changing hemodynamic conditions[7]. Therefore, a more thorough understanding of the biological composition and biomechanics of the vascular system is warranted. There is a need for experimental data with an effort to determine the response of the aorta to a pulsatile waveform. Biological tissues, though subject to conservation of mass, momentum and energy, have unique constitutive equations that differentiate them from inorganic materials, and thus make them harder to characterize. This review aims to present, in brief, the current state of the biomechanical characterization of arterial walls, particularly the aorta, through discussion of testing methods and their findings. Moreover, overarching concepts such as histological effects, age dependent effects, segmental effects, hemodynamic effects, viscoelastic modelling and torsion will be briefly explored.
Current testing methods
Uniaxial tensile test
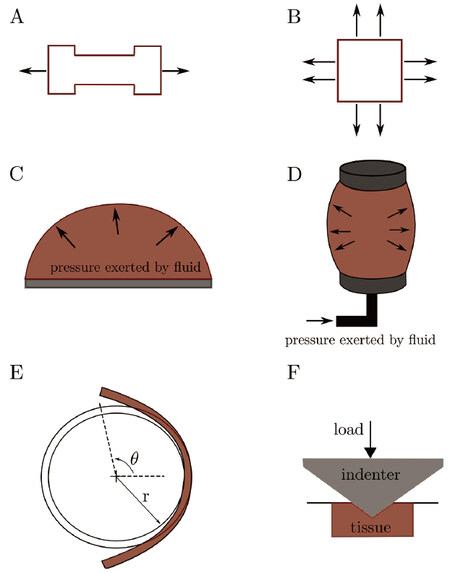
Uniaxial tensile tests have been widely used to test biological tissues in vitro due to their simplicity and ability to give precise information regarding local properties of soft tissues. While extending a piece of the sample (either rectangular in shape, or dog-bone shaped), the displacement and resulting force are recorded until fracture [Figure 1A]. This data can be used to obtain a variety of stress-strain relations, and depending on the constitutive model chosen, authors may make use of different stress and strain parameters outlined throughout Continuum Mechanics such as Cauchy stress, engineering stress, Second Piola-Kirchoff stress, Green strain, true strain, and engineering strain.
Figure 1. Schematics of the mechanical tests described for aortic mechanics where A: shows a uniaxial test; B: biaxial testing; C: the bulge-inflation test; D: depicts inflation-extension testing; E: opening angle testing: Upon cutting an intact circumferential segment of an artery in an unloaded state (described by radius, r), an expansion of the segment is observed over time, leading to an equilibrium zero-stress state (described by radius, R, and opening angle, theta). The residual strains can be obtained from comparison between the two states; F: nano-indentation test: an indenter of known material properties is pressed into the tissue with a known loading pattern, after which the area of the indentation is observed, and the hardness of the material can be calculated
With uniaxial tensile testing, one can obtain the ultimate tensile strength (strength most often recorded at failure), the yield strength, as well as the strain at failure. A single value of Young’s modulus, which is useful for defining non-biological materials, cannot be adequately used for blood vessels since they are characterized by a non-linear elastic behaviour. A common method to define this parameter for biological tissues is to calculate the modulus incrementally and report it for a specified range of stress. Additionally, most authors make use of the maximum tangential modulus to describe vessel stiffness and make succinct comparisons between intra-study specimens. Currently there is no standard for testing protocol, and variations in experimental parameters such as the force range and number of cycles for preconditioning, loading or strain rate, or the strain measure used to calculate elasticity parameters, make lateral comparisons between papers difficult at best[4,8].
A focus of uniaxial testing on the aneurysmal aorta has been considering the region- and layer-dependent variations of the wall behaviour. Iliopoulos et al.[9] first showed that the ascending thoracic aortic aneurysm (ATAA) exhibits heterogeneity between the anterior, posterior, right and left lateral regions. Note that the following studies presented in this section divided specimens into these same four regions. The data from the circumferential direction suggested this direction to be stiffer than the longitudinal direction at physiological and high stresses. While no differences in the peak elastic modulus were observed between the four regions in the circumferential direction, the longitudinal direction results indicated that the anterior region was significantly less stiff than the other regions. The results showed that no correlation existed between failure stress and the diameter of the whole ATAA[9]. The layer-specific differences of the tunica intima, media, and adventitia from uniaxial data of the ATAA were documented by Sokolis et al.[10]. In general, the circumferential stiffness was recorded as higher than the longitudinal direction in the adventitia and media, but not the intima, though all layers had a higher failure stress in the circumferential direction. This is true for all four regions (previously introduced) of the media, but only for the anterior and posterior regions of the adventitia. This study also brought up the important concern that the adhesive connections between the three layers needs to be considered when drawing conclusions regarding the overall mechanical properties of the wall from those of the individual layer behaviour. Khanafer et al.[11] performed uniaxial tests on the ATAA and highlighted the importance of the elastic modulus in assessing risk of aneurysm rupture. Sassani et al.[12] examined the regional uniaxial response (collecting bi-dimensional strain data) of the intimal, medial, and adventitial layers of the aneurysmal ascending aorta in the circumferential and longitudinal directions, again separating the specimens into four regions. A decoupled microstructure-based formulation, resulting in a reduced two-fiber model, was used to describe the uniaxial behaviour in either direction, concluding that material parameters are highly dependent on differences within the underlying wall composition. Further studies on these histological effects are presented under the section titled Factors Affecting Arterial Mechanics.
Biaxial tensile test
While uniaxial tests describe failure properties of vessel walls adequately, as shown by the studies cited in the previous section, characterising loading response along a singular axis does not reflect in vivo conditions, where the blood vessel wall is subject to multi-axial stresses. Guinea et al.[13] performed uniaxial tests on healthy thoracic aortas, with samples obtained from persons deceased from non-cardiovascular causes. They acknowledged the limitations of the uniaxial test, stating that it poorly reproduces the complex loading conditions seen at the physiological level, which are better described by biaxial testing. In addition, uniaxial testing does not provide a true understanding of the anisotropic properties of the vessel wall, as the circumferential and axial test strip come from different locations. Biaxial testing is done with a square sample, which is placed in a loading rig with four arms, generally by means of hooks, at 90 degrees to each other [Figure 1B]. A significant volume of literature has focused on biaxial testing of both healthy and diseased tissue[14-18]. Alreshidan et al.[19] used biaxial testing on resected ascending aortic tissue to compare to and validate the use of in vivo speckle tracking transesophageal echocardiography to estimate aortic stiffness to better stratify the risk of aortic aneurysm rupture. The advancement of in vivo imaging techniques gives rise to more reliable data; however, the behaviour of the aorta with these tests is limited to patient-specific physiological conditions. In addition, in vivo testing presents multivariate data, as it is not possible to disregard other non-loading effects, such as the effect of surrounding perivascular adipose tissue. Drawbacks of biaxial testing arise from the attachment method, i.e., the failure of the tissue usually occurs at the attachment location and rupture is also influenced by the components used to hold the tissue[20]. In addition, testing samples of various shapes does not allow for accurate comparison since the geometry, in addition to the clamping method, can alter the mechanical properties observed, as was described by Waldman et al.[21].
Bulge inflation test
The bulge inflation test, also referred to as the membrane bulge test, gives information on biaxial behaviour, and was used most recognizably in the work by Mohan and Melvin[22] on healthy human thoracic aorta specimens. This test involves using a square specimen, which is secured in the inflation device through an airtight seal [Figure 1C]. A fluid, generally water, is released at a specified rate, and the expansion of the specimen is tracked optically with complex digital imaging techniques to obtain the strain field. This technique has also been applied to analyze the mechanical behaviour of aneurysms[5,23,24]. Romo et al.[23] utilized the bulge inflation test on the ascending aorta to observe the most likely location of aneurysmal rupture many stages of deformation before the rupture took place by noting the region that had the most amount of localized thinning. They created local thickness over pressure maps to predict the site of aneurysm rupture and they posited that the site of rupture mostly occurs not at the location of maximum stress but at the location of greatest wall weakening.
Inflation-extension test
An inflation-extension test differs from the aforementioned techniques in that it replicates the in vivo cylindrical loading scenario of a blood vessel. In general, an intact portion of a blood vessel is extended by some means to replicate axial loading, and fluid is run through the conduit to enact circumferential loading [Figure 1D]. Optical methods may be used to track displacement and force of pre-set markers on the blood vessel[25,26]. Courtial et al.[27] present, in detail, an inflation device that was coupled with non-invasive imaging techniques such as ultrasound, in order to identify the parameters of a silicone tube based hyperviscoelastic model that represented the native aorta. They found that the inflation-extension test was adequate in validating this model. The inflation-extension property of the aorta was further tested by Horny et al.[28] in an analytical simulation, using parameters from autopsy measurements. They established that while axial pre-stretch, which allows the aorta to minimize deformation during systole and diastole, decreases with age, the prespecified value of axial pre-stretch can still have a significant effect on the mechanical properties of the vessel wall. Inflation-extensions tests are valuable for understanding the operating mode of arteries. However, inverse analysis and simplification is typically required to find the stress-strain relationship from the pressure-diameter measurements[13].
Opening angle test
Residual stresses are present in the circumferential direction of the arterial wall, and this can be visually shown by cutting open a ring segment of an artery along the axial direction[25,26]. Subsequently the opening angle formed by the specimen may be optically measured until the cut section stabilizes [Figure 1E]. Accounting for these residual stresses, which have been shown to be present in the axial direction as well[29], leads to a significantly lower circumferential stress gradient along the thickness of the vessel[30]. This observation is better explained when considering the circumferential residual strains, wherein accounting for these strains works to decrease the stretch ratio at the inner surface, while increasing that of the outer surface[31,32]. In essence, to account for residual stresses, “state 0” (stress-free state) is taken as the reference configuration, rather than “state 1” (unloaded state - outlined in Ref. [30]). Moreover, the presence of residual stresses in arteries results in a more uniform stress distribution throughout the vessel wall[33,34]. Zheng and Ren[35] further explored the effects of three-dimensional residual stresses in each of the three arterial layers. They showed that the residual circumferential stress is compressive within the intima, while tensile in the remaining two layers, and that the bending of the media in the longitudinal direction noticeably affects the mechanical behavior of the arterial wall. Cardamone et al.[36] attribute the origin of this residual stress to non-uniform growth and remodelling as well as temporo-spatial variances in wall components.
Sokolis et al.[37] examined the regional distribution of circumferential residual strains in the human aorta according to age and gender to gain a more detailed understanding of the zero-stress and no-load states of the human aorta. The opening angle measurement, which characterizes the circumferential residual strain, was highest in the aortic arch and declined in the descending aorta. Opening angle measurements for aneurysmal tissue are difficult to obtain due to the irregular shape of the diseased vessel wall. Sokolis[38] discussed the residual stresses within the ATAA in great depth, presenting several important conclusions regarding the vessel wall behaviour: (1) change in the wall composition, mainly the decreased presence of elastin within the aneurysmal vessel wall, results in greater viscoelastic behaviour; (2) residual strains vary along the circumferential direction within each of the three layers of the wall; and (3) analysing the residual stresses of the layers individually reveals that the intima is held in compression, while the media is in tension. Contrary to previous studies performed on the abdominal aorta, the adventitia was shown to be under compression in the circumferential direction, though still under tension longitudinally. Higher opening angles were measured in the aneurysmal tissue and it was postulated that this might be a compensatory mechanism to increase the vessel’s resistance to dissection[39]. This is perhaps similar to a compensatory increase in opening angle measurements with increased stiffness of the aorta with age. Most recently, Sokolis et al.[40] employed opening angle testing to study the axial residual strain variations in the human aorta according to age and gender. Interestingly, they found that the axial opening angle and residual stress decreased with age as compared to the circumferential residual strain which had increased with age.
Nanoindentation test
Nanoindentation tests may be used to characterize local deformation and properties in multilayer materials [Figure 1F]. Though it is known that the artery is made up of three distinct layers, a large portion of the aorta’s biomechanical modelling describe it as a single, homogenous layered vessel. That being said, experimental results regarding the behaviour of the individual layers has been performed on ATAA tissue in detail, and strain-energy-functions were fit to the data to describe the behaviour. Reiterating the discussion from Uniaxial Testing, it was shown that the intima was the weakest of the three layers, with the adventitia exhibiting the highest failure stresses and resistance to excessive deformation, preventing rupture. Sokolis et al.[10] revealed that the behaviour of the intact aneurysmal wall was most similar to the intima and media layers, however, drawing inferences regarding the overall layered vessel wall behaviour remains a challenge due to the complex attachment and interaction between layers.
Hemmasizadeh et al.[41] introduced a custom nanoindentation technique showing that the inner layer is more compliant than the outer layer and sustains higher strains. Therefore, rupture of the aorta travels outwards from the inner layer. This is verified by Manopoulos et al.[42] by uniaxial tests performed on ascending thoracic aortic dissected tissue to understand the failure properties for the vessel wall as divided into an inner (intima-media) and outer (media-adventitia) layer. They found that the inner layer displayed a significantly lower strength than the outer layer, in addition to a sharp decline of stress following rupture, unlike the outer layer which exhibited a slow decrease to zero stress. Interestingly, the relative strengths of the two layers differed significantly in magnitude from what has previously been reported on the individual layers[10,42]. The outer layer was shown to be stronger than the adventitia alone, and in comparing the properties of the origin of inner layer failure to distal locations, it was evident that dissection occurred where the layer was thinner and exhibited increased stiffness. These results offer valuable insight as to why the specimens studied[42] dissected, rather than undergoing full rupture.
Factors affecting arterial mechanics
Histological effects
Soft tissues, including blood vessels, contain collagen, elastin, and ground substance. The relative amount, density, and arrangement of these three constituents greatly affect the mechanical response of the tissue[43,44]. Therefore, it is important to consider histological composition when characterizing the physical properties of the aortic wall. Distribution of elastic fibres in soft tissues and its relation to mechanical properties have been studied extensively[43,45]. Bellini et al.[18] better defined the mechanical environments of the media and adventitia layers within the arterial wall by highlighting the roles that smooth muscle cells and fibroblasts play in arterial homeostasis. Taghizadeh et al.[46] evaluated the mechanical properties of the aortic wall while accounting for the lamellar structure within the media layer. Cardamone et al.[36] confirmed that elastin contributes significantly to the shortening of arteries observed upon cutting along the circumferential direction (residual stresses), and to the opening angle phenomenon. Holzapfel et al.[47] combined histological data with computational modelling to create a general constitutive model of the anisotropic collagen fibre dispersion in arterial walls. In terms of aneurysmal tissue, studies focusing on the ascending aorta report reduced levels of elastin, but normal levels of collagen, throughout the vessel wall when compared to a healthy aorta[48,49]. Aneurysm development in the ascending aorta has been shown to be associated with higher stiffness of the wall, resulting in increased wall stresses, but not leading to a weakening of the wall for age-matched subjects[48].
Segmental effects
There are segmental differences in the structure and mechanical properties of the aorta, and these are dependent on the magnitude of stress that the wall is subjected to regularly under normal conditions. Schriefl et al.[50] incorporated the themes of segmental and histological analysis to study the layer specific distribution and orientation of collagen fibres in the abdominal and thoracic aorta. They concluded that there were distinct fibre families, directions and dispersion present in the three arterial layers: these variations between the layers underlie their different mechanical and functional properties. Sassani et al.[12] utilized a four fiber microstructure based model to characterize region and layer-specific material properties in ascending aortic aneurysmal tissue samples; their novel hypothesis that the fibers are able to support compressive forces provides further insight into the role of elastin and collagen in withstanding mechanical loads. The aortic wall shows an increase in viscoelastic creep further from the aortic root, which can be attributed to a decrease in elastin content. Irregular variability in the opening angle was observed over the length of the vessel, the pattern of which was determined to be similar across both young (less than 40 years of age) and old subjects[37]. Haskett et al.[14] delved into the critical importance of gaining a better structural model of the aorta through quantification of microstructural and mechanical changes. Specifically, they looked at anisotropy and extra-cellular matrix microstructure changes as a function of location and age of the aorta.
Age-dependent effects
Another concept of interest is the age-dependent elastic behaviour of the arterial wall which is closely related to the histological compositional changes that occur with age. These alterations with age are explored by Maceri et al.[51]; they introduce a multiscale mechanical model that accounts for nanoscale effects of cross-link stretching, as well as micro- and macroscale mechanisms. This model further supports experimental evidence regarding the histological alterations in the aortic wall that occur with age, showing evidence between the influence of cross-link density and stiffness on the elastic modulus. With age, there is an increase in aortic diameter, thickness, elastin stiffness and collagen cross links and there is a decrease in collagen amount, collagen fiber radius and waviness. Carallo et al.[52] concur and describe the effects of ageing as a change in the arterial wall with an increased collagen presence and reduced elastin function, leading to a larger and stiffer vessel. Moreover, this increase in aortic stiffness and thickness is greatest circumferentially[14].
Cavalcante et al.[53] present a comprehensive review on arterial stiffness associated with age and, in particular, the acceleration of aortic stiffness due to hypertension. They identify arterial stiffness as an important prognostic factor and emphasize its deleterious effect on the Windkessel function of the aorta. Moreover, they identify pharmacological and non pharmacological therapies targeted against increased aortic stiffness. This further highlights the importance of identifying contributing factors that affect vessel function so that more targeted therapies can be employed. A monotonic decrease in circumferential and axial tensile strength of the arterial wall with age is evident as well[13,54,55]. Guinea et al.[13] utilized uniaxial tensile tests on donor thoracic aortas and found that the tensile strength of the thoracic aorta falls rapidly after age 30 and Morrison et al.[55] found that circumferential and longitudinal strain decreased by 50% with increasing age (patients with a mean age of 68 compared to patients with a mean age of 41). Deveja et al.[56] found a negative correlation between failure stress and stretch with increasing age in all three layers of the ascending aorta and a similar correlation was found by Iliopoulos et al.[57] in tissue samples of patients with Sinus of Valsalva aneurysms. Tracy and Eigenbrodt[58] found that a disproportionate increase in vessel wall thickness with age causes a deviation from the Laplace law: they introduced age specific constants into the Laplace equation to make their data conform to Laplace expectations. This further highlights the need for a more comprehensive guideline to assess aneurysm rupture risk, especially in an ageing population, than the current diameter-based guidelines which were formed on the basis of the Laplace Law.
Hemodynamic effects
Hemodynamics is of particular interest as a potential factor in arterial disease formation and progression. Dynamic in vitro tests could show the effect of flow on the healthy arterial structure and subsequent disease formation while still allowing for control of the loading conditions and isolating mechanical effects. Pulsatile arterial hemodynamics has been explored in numerous studies[52,59,60]. The scope of this review is not intended to cover studies on hemodynamics and associated arterial mechanics; however, to fully understand the complexity of characterizing the vasculature wall and the mechanisms that may lead to cardiovascular complications, the fluid-structure interactions - for example, wave propagation in arterial walls, local hemodynamics, and temporal wall shear stress - should be considered. Therefore, in brief and without discussion of methods employed, several key concepts are mentioned. While the flow of blood is generally laminar, in some cases this flow can be disrupted and result in transitional conditions [Supplementary Figure 1], which may contribute to certain cardiovascular pathologies. Transitional flow in the presence of hypercholesterolemia has been proven to prime the vessel wall for the pathogenesis of atherosclerosis[61]. Transitional blood flow has also been suggested as a cause of post-stenotic dilatation, however this association may be due to the common occurrence of turbulence alongside stenosis of the blood vessel wall[62,63].
Transition has been proven to significantly increase pressure and shear stress within aneurysmal regions. Khanafer et al.[11,64] suggest that this may result in a self-perpetuating mechanism of further dilatation and subsequent increase of turbulence in the region. It has been suggested that the associated hemodynamics through an aneurysm, such as recirculating flow, may result in the formation of thrombi[64,65]. The majority of the literature; however, supports the hypothesis that the formation of an aneurysm is a multi-factorial, degenerative process[66], not solely affected by hemodynamics and mechanical wall stress, but including inflammation and immune response, molecular genetics, and degradation of surrounding connective tissue[64].
Viscoelastic modelling
Large arteries are viscoelastic, which entails distinct mechanical behaviour compared to typical elastic models and calls for analysis of time-dependent behaviour. Due to this viscous component, there is energy retained within the arterial wall upon unloading, which is seen through hysteresis present in the stress-strain, and pressure-diameter curves of arteries[67,68]. Hysteresis loops may be used to estimate damping capacity, which is associated with the ratio of the dissipated energy to the stored energy[69]. An interesting study on strain-rate effect of mechanical properties by Delgadillo et al.[70] revealed that at a stretch ratio of 1.5, the experienced load within the arterial wall is reduced by 20% when the strain rate is increased from 10 to 200 %/S. They suggested that: “this behavior might be a consequence of the faster fluidization and small re-solidification that occurs in the cell at higher deformation rates”.
Torsion
While the response of arteries to axial stretching and circumferential stretching has long been studied and used to quantify failure of the arterial wall, the effect of torsion has been explored to a lesser extent, despite the fact that in vivo, arteries are often subject to twisting along the longitudinal direction with body movement[71,72]. Klein et al.[72] found that there was a significant change in arterial length, curvature and twist in the femoropopliteal arteries when subjects were cross legged compared to straight legged. Furthermore, the abdominal aorta and common iliac arteries exhibit significant morphological deformations from musculoskeletal motion. Hence, torsion is of particular concern since it has been identified as a possible contribution to failure of stents in the more mobile arteries[71-74]. Further study on the shortening, twisting and bending patterns of these arteries with stenting is required.
Conclusion
The inter-individual heterogeneity of the aorta’s geometry and composition, and the distinct differences in regional mechanical properties[26], fuel the difficulty behind understanding the underlying mechanics of the aorta. Uniaxial tests provide data regarding local mechanical properties and provide base comparisons between diseased and healthy arteries[4,8-10,12,75]. However, biaxial tests provide a better estimate of the multiaxial and anisotropic properties of arteries[14-18]. Both tests allow for data collection of incremental elastic modulus until specimen failure, thus providing rudimentary data about rupture conditions. Bulge inflation tests further provide information on biaxial behaviour[5,22-24]; however, inflation-extensions tests replicate in vivo loading scenarios and are more suited to explore the in-depth loading mechanisms under which healthy tissue may become diseased[26-29]. The opening angle test illustrates the residual stresses that are present in the circumferential direction in arterial walls[25,26,29,37,39]. Lastly, the nanoindentation test may be used to characterize local deformation and inter-layer properties of arteries[10,41]. There has been great effort put into quantification of the mechanical properties of the aorta; however, acquiring whole tissue is difficult and the importance of standard testing cannot be overlooked. The development of more robust in vitro test methods and further research with respect to spatial and temporal variations in aortic composition will lead to a clearer understanding of the biomechanical contributions to vascular pathologies such as aneurysms. This will potentially lead to a more comprehensive stratification of aneurysm rupture risk and will hopefully lead to more targeted treatment strategies.
Declarations
Authors’ contributionsPerformed literature review and composed manuscript: Pejcic S, Hassan SMA
Edited Manuscript: Rival DE, Bisleri G
Availability of data and materialsThe data was sourced via Queen’s Summon Search and PubMed.
Financial support and sponsorshipSP acknowledges support from the Natural Sciences and Engineering Research Council (NSERC) Canadian Graduate Scholarship - Master’s Program.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2019.
REFERENCES
1. Kontopodis N, Metaxa E, Papaharilaou Y, Tavlas E, Tsetis D, et al. Advancements in identifying biomechanical determinants for abdominal aortic aneurysm rupture. Vascular 2015;23:65-77.
2. Emmott A, Garcia J, Chung J, Lachapelle K, El-Hamamsy I, et al. Biomechanics of the Ascending Thoracic Aorta: A Clinical Perspective on Engineering Data. Can J Cardiol 2016;32:35-47.
3. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926.
4. Avanzini A, Battini D, Bagozzi L, Bisleri G. Biomechanical evaluation of ascending aortic aneurysms. Biomed Res Int 2014;2014:820385.
5. Trabelsi O, Davis FM, Rodriguez-Matas JF, Duprey A, Avril S. Patient specific stress and rupture analysis of ascending thoracic aneurysms. J Biomech 2015;48:1836-43.
6. Boodhwani M, Andelfinger G, Leipsic J, Lindsay T, McMurtry MS, et al. Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol 2014;30:577-89.
7. Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol 1992;263:C389-96.
8. Sokolis DP. Passive mechanical properties and structure of the aorta: segmental analysis. Acta Physiol (Oxf) 2007;190:277-89.
9. Iliopoulos DC, Deveja RP, Kritharis EP, Perrea D, Sionis GD, et al. Regional and directional variations in the mechanical properties of ascending thoracic aortic aneurysms. Med Eng Phys 2009;31:1-9.
10. Sokolis DP, Kritharis EP, Iliopoulos DC. Effect of layer heterogeneity on the biomechanical properties of ascending thoracic aortic aneurysms. Med Biol Eng Comput 2012;50:1227-37.
11. Khanafer K, Duprey A, Zainal M, Schlicht M, Williams D, et al. Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. J Thorac Cardiovasc Surg 2011;142:682-6.
12. Sassani SG, Tsangaris S, Sokolis DP. Layer-and region-specific material characterization of ascending thoracic aortic aneurysms by microstructure-based models. J Biomech 2015;48:3757-65.
13. Guinea GV, Atienza JM, Rojo FJ, García-Herrera CM, Yiqun L, et al. Factors influencing the mechanical behaviour of healthy human descending thoracic aorta. Physiol Meas 2010;31:1553-65.
14. Haskett D, Johnson G, Zhou A, Utzinger U, Vande Geest J. Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomech Model Mechanobiol 2010;9:725-36.
15. Geest J, Sacks MS, Vorp D. The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J Biomech 2006;39:1324-34.
16. Zemánek M, Burša J, Děták M. Biaxial tension tests with soft tissues of arterial wall. Eng Mechanics 2009;16:3-11.
17. Azadani AN, Chitsaz S, Matthews PB, Jaussaud N, Leung J, et al. Comparison of mechanical properties of human ascending aorta and aortic sinuses. Ann Thorac Surg 2012;93:87-94.
18. Bellini C, Ferruzzi J, Roccabianca S, Di Martino ES, Humphrey JD. A microstructurally motivated model of arterial wall mechanics with mechanobiological implications. Ann Biomed Eng 2014;42:488-502.
19. Alreshidan M, Shahmansouri N, Chung J, Lash V, Emmott A, et al. Obtaining the biomechanical behavior of ascending aortic aneurysm via the use of novel speckle tracking echocardiography. J Thorac Cardiovasc Surg 2017;153:781-8.
20. Marra SP, Kennedy FE, Kinkaid JN, Fillinger MF. Elastic and rupture properties of porcine aortic tissue measured using inflation testing. Cardiovasc Eng 2006:6123-31.
21. Waldman S, Sacks MS, Lee J. Boundary conditions during biaxial testing of planar connective tissues Part II Fiber orientation. J Mater Sci Mater Med 2002;21:1215-21.
22. Mohan D, Melvin J. Failure properties of passive human aortic tissue. II-Biaxial tension tests. J Biomech 1983;16:31-44.
23. Romo A, Badel P, Duprey A, Favre JP, Avril S. In vitro analysis of localized aneurysm rupture. J Biomech 2014;47:607-16.
24. Kim JH, Avril S, Duprey A, Favre JP. Experimental characterization of rupture in human aortic aneurysms using a full-field measurement technique. Biomech Model Mechanobiol 2012;11:841-53.
25. Labrosse MR, Beller CJ, Mesana T, Veinot JP. Mechanical behavior of human aortas: experiments, material constants and 3-D finite element modeling including residual stress. J Biomech 2009;42:996-1004.
26. Labrosse MR, Gerson ER, Veinot JP, Beller CJ. Mechanical characterization of human aortas from pressurization testing and a paradigm shift for circumferential residual stress. J Mech Behav Biomed 2013;17:44-55.
27. Courtial EJ, Orkisz M, Douek PC, Huet L, Fulchiron R. Identifying hyper-viscoelastic model parameters from an inflation-extension test and ultrasound images. Exp Mech 2015;55:1353-66.
28. Horný L, Netušil M, Voňavková T. Axial prestretch and circumferential distensibility in biomechanics of abdominal aorta. Biomech Model Mechanobiol 2014;13:783-99.
29. Wang R, Gleason RL Jr. A mechanical analysis of conduit arteries accounting for longitudinal residual strains. Ann Biomed Eng 2010;38:1377-87.
30. Chaudhry HR, Bukiet B, Davis A, Ritter AB, Findley T. Residual stresses in oscillating thoracic arteries reduce circumferential stresses and stress gradients. J Biomech 1997;30:57-62.
31. Chuong CJ, Fung YC. Three-dimensional stress distribution in arteries. J Biomech Eng 1983;105:268-74.
33. Fung YC. What are the residual stresses doing in our blood vessels? Ann Biomed Eng 1991;19:237-49.
35. Zheng X, Ren J. Effects of the three-dimensional residual stresses on the mechanical properties of arterial walls. J Theor Biol 2016;393:118-26.
36. Cardamone L, Valentín A, Eberth JF, Humphrey JD. Origin of axial prestretch and residual stress in arteries. Biomech Model Mechanobiol 2009;8:431-46.
37. Sokolis DP, Savva GD, Papadodima SA, Kourkoulis SK. Regional distribution of circumferential residual strains in the human aorta according to age and gender. J Mech Behav Biomed Mater 2017;67:87-100.
39. Sokolis DP. Effects of aneurysm on the directional, regional, and layer distribution of residual strains in ascending thoracic aorta. J Mech Behav Biomed Mater 2015;46:229-43.
40. Sokolis DP, Bompas A, Papadodima S, Kourkoulis SK. Variation of Axial Residual Strains Along the Course and Circumference of Human Aorta Considering Age and Gender. J Biomech Eng 2019; doi: 10.1115/1.4043877.
41. Hemmasizadeh A, Autieri M, Darvish K. Multilayer material properties of aorta determined from nanoindentation tests. J Mech Behav Biomed 2012;15:199-207.
42. Manopoulos C. Identification of regional/layer differences in failure properties and thickness as important biomechanical factors responsible for the initiation of aortic dissections. J Biomech 2018;80:102-10.
43. Marino M, Vairo G. Multiscale elastic models of collagen bio-structures: from cross-linked molecules to soft tissues. Comput Method Biomec 2013;14:73-102.
44. Brüel A, Ortoft G, Oxlund H. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis 1998;140:135-45.
45. Akhtar R, Schwarzer N, Sherratt MJ, Watson RE, Graham HK, et al. Nanoindentation of histological specimens: mapping the elastic properties of soft tissues. J Mater Res 2009;24:638-46.
46. Taghizadeh H, Tafazzoli-Shadpour M, Shadmehr MB, Fatouraee N. Evaluation of biaxial mechanical properties of aortic media based on the lamellar microstructure. Materials (Basel) 2015;8:302-16.
47. Holzapfel GA, Niestrawska JA, Ogden RW, Reinisch AJ, Schriefl AJ. Modelling non-symmetric collagen fibre dispersion in arterial walls. J R Soc Interface 2015;12:20150188.
48. Iliopoulos DC, Kritharis EP, Giagini AT, Papadodima SA, Sokolis DP. Ascending thoracic aortic aneurysms are associated with compositional remodeling and vessel stiffening but not weakening in age-matched subjects. J Thorac Cardiov Sur 2009;137:101-9.
49. Sokolis DP, Kritharis EP, Giagini AT, Lampropoulos KM, Papadodima SA, et al. Biomechanical response of ascending thoracic aortic aneurysms: association with structural remodelling. Comput Method Biomec 2012;15:231-48.
50. Schriefl AJ, Zeindlinger G, Pierce DM, Regitnig P, Holzapfel GA. Determination of the layer-specific distributed collagen fibre orientations in human thoracic and abdominal aortas and common iliac arteries. J R Soc Interface 2011;9:1275-86.
51. Maceri F, Marino M, Vairo G. Age-dependent arterial mechanics via a multiscale elastic approach. Int J Numer Meth Eng 2013;14:141-51.
52. Carallo C, Irace C, Pujia A, De Franceschi MS, Crescenzo A, et al. Evaluation of common carotid hemodynamic forces. Relations with wall thickening. Hypertension 1999;34:217-21.
53. Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 2011;57:1511-22.
54. García-Herrera CM, Atienza JM, Rojo FJ, Claes E, Guinea GV, et al. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med Biol Eng Comput 2012;50:559-66.
55. Morrison TM, Choi G, Zarins CK, Taylor CA. Circumferential and longitudinal cyclic strain of the human thoracic aorta: age-related changes. J Vasc Surg 2009;49:1029-36.
56. Deveja RP, Iliopoulos DC, Kritharis EP, Angouras DC, Sfyris D, et al. Effect of aneurysm and bicuspid aortic valve on layer-specific ascending aorta mechanics. Ann Thorac Surg 2018;106:1692-701.
57. Iliopoulos DC, Kritharis EP, Boussias S, Demis A, Iliopoulos CD, et al. Biomechanical properties and histological structure of sinus of Valsalva aneurysms in relation to age and region. J Biomech 2013;46:931-940.
58. Tracy RE, Eigenbrodt ML. Coronary artery circumferential stress: departure from Laplace expectations with aging. ScientificWorldJournal 2009;9:946-60.
59. Taylor CA, Steinman DA. Image-based modeling of blood flow and vessel wall dynamics: applications, methods and future directions: sixth international bio-fluid mechanics symposium and workshop, March 28-30, 2008 Pasadena, California. Ann Biomed Eng 2010;38:1188-203.
60. Cebral JR, Duan X, Chung BJ, Putman C, Aziz K, et al. Wall mechanical properties and hemodynamics of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2015;36:1695-703.
61. Prado CM, Ramos SG, Elias J, Rossi MA. Turbulent blood flow plays an essential localizing role in the development of atherosclerotic lesions in experimentally induced hypercholesterolaemia in rats. Int J Exp Pathol 2008;89:72-80.
62. Zaroff LI, Kreel I, Sobel HJ, Baronofsky ID. Multiple and infraductal coarctations of the aorta. Circulation 1959;20:910-7.
64. Khanafer KM, Bull JL, Upchurch GR, Berguer R. Turbulence significantly increases pressure and fluid shear stress in an aortic aneurysm model under resting and exercise flow conditions. Ann Vasc Surg 2007;21:67-74.
65. Les AS, Shadden SC, Figueroa CA, Park JM, Tedesco MM, et al. Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann Biomed Eng 2010;38:1288-313.
67. London GM, Pannier B. Arterial functions: how to interpret the complex physiology. Nephrol Dial Transplant 2010;25:3815-23.
68. Tian L, Wang Z, Lakes RS, Chesler NC. Comparison of approaches to quantify arterial damping capacity from pressurization tests on mouse conduit arteries. J Biomech Eng 2013;135:54504.
69. Rosset E, Brunet C, Rieu R, Rolland P, Pellissier JF, et al. Viscoelastic properties of human arteries methodology and preliminary results. Surg Radiol Anat 1996;18:89-96.
70. Delgadillo JOV, Delorme S, Mora V, DiRaddo R, Hatzikiriakos SG. Effect of deformation rate on the mechanical properties of arteries. J Biomed Sci Engine 2010;3:124-37.
71. Cheng CP, Wilson NM, Hallett RL, Herfkens RJ, Taylor CA. In vivo MR angiographic quantification of axial and twisting deformations of the superficial femoral artery resulting from maximum hip and knee flexion. J Vasc Interv Radiol 2006;17:979-87.
72. Klein AJ, Chen SJ, Messenger JC, Hansgen AR, Plomondon ME, et al. Quantitative assessment of the conformational change in the femoropopliteal artery with leg movement. Catheter Cardiovasc Interv 2009;74:787-98.
73. Vos AW, Linsen MA, Marcus JT, van den Berg JC, Vos JA, et al. Carotid artery dynamics during head movements: a reason for concern with regard to carotid stenting? J Endovasc Ther 2003;10:862-9.
74. Choi G, Shin LK, Taylor CA, Cheng CP. In vivo deformation of the human abdominal aorta and common iliac arteries with hip and knee flexion: implications for the design of stent-grafts. J Endovasc Ther 2009;16:531-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Pejcic S, Hassan SMA, Rival DE, Bisleri G. Characterizing the mechanical properties of the aortic wall. Vessel Plus 2019;3:32. http://dx.doi.org/10.20517/2574-1209.2019.18
AMA Style
Pejcic S, Hassan SMA, Rival DE, Bisleri G. Characterizing the mechanical properties of the aortic wall. Vessel Plus. 2019; 3: 32. http://dx.doi.org/10.20517/2574-1209.2019.18
Chicago/Turabian Style
Pejcic, Sonja, Syed M. Ali Hassan, David E. Rival, Gianluigi Bisleri. 2019. "Characterizing the mechanical properties of the aortic wall" Vessel Plus. 3: 32. http://dx.doi.org/10.20517/2574-1209.2019.18
ACS Style
Pejcic, S.; Hassan SMA.; Rival DE.; Bisleri G. Characterizing the mechanical properties of the aortic wall. Vessel Plus. 2019, 3, 32. http://dx.doi.org/10.20517/2574-1209.2019.18
About This Article
Copyright
Data & Comments
Data
 Cite This Article 25 clicks
Cite This Article 25 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.