Atrial septal defect repair in the age of transcatheter devices
Abstract
The aim of this review is to discuss the management of atrial septal defects (ASD) in the adult patient paying special attention to the elderly population and the most recent transcatheter advancements. ASDs are characterized by the following categories: ostium secundum, ostium primum, sinus venosus, and coronary sinus defects; though multiple defects may exist concurrently. Intervention for closure of ASDs are indicated with the development of right ventricular volume overload, or in the clinical context of paradoxical embolic stroke. Previously, there was significant disagreement regarding the timing of ASD closure in adult patients, but there is now general consensus that adult patients with clinical evidence of right ventricular overload should undergo closure of ASDs at the time of presentation. The present review describes the typical presentation of patients with symptomatic ASD’s, medical management, and whether surgical or percutaneous approach should be pursued. We will also discuss other important considerations for patient selection and potential early and late complications of transcatheter ASD closure such as congestive heart failure, device embolization, and tissue erosion. At the time of this writing, there are currently three FDA-approved devices for percutaneous VSD closure including the AmplatzerTM Septal Occluder (ASO, St. Jude Medical, St. Paul, MN), Gore HELEXTM Septal Occluder (W.L. Gore and Associates, Newark, NJ), and Gore CARDIOFORMTM Septal occluder (GCSO, W.L. Gore and Associates, Newark, NJ) devices. Many premarket approvals were granted for devices that never went to market due to poor investigational study performance. Likewise, the HELEX device has since been discontinued upon bringing the GCSO device to market. We will focus primarily on the ASO device with a brief review of current investigations into the GCSO device, both of which carry an indication for closure small to medium sized ASDs in the ostium secundum position. Additionally, this review covers the safety of transcatheter closure of ASDs with currently available devices, review studies associated with devices available outside the United States, and perioperative considerations for transcatheter intervention. Obstacles to device employment and countermeasures to overcome operational challenges will also be discussed. To this end, variations or similarities of currently approved devices will be emphasized throughout this discussion where possible. Lastly, we will offer insights into device evolution trends with the expectation of new device developments on the horizon. We will briefly discuss up and coming areas of active research, including the emerging fields of novel biomaterials and gene therapy.
Keywords
Introduction
Atrial septal defects (ASD) are one of the most common congenital cardiac abnormalities reported both in adolescent and adult populations. The incidence of newly diagnosed atrial septal defects are second only to bicuspid aortic valves as the most common congenital heart disease in children, with ASDs accounting for the majority of congenital malformations diagnosed in adults[1]. ASDs may be detected in asymptomatic patients, though physical findings may be subtle at best making detection prior to associated symptoms difficult in most clinical settings[2]. Though patients with ASDs may remain asymptomatic well into adulthood, undetected ASDs may lead to potentially irreversible complications such as arrhythmias, pulmonary hypertension, stroke or their associated sequelae[3,4]. The true incidence of ASD may be significantly underestimated due to the nature of their relatively silent clinical course. One study estimates 941 per one million live births have an ASD based on the metanalysis of 43 studies, which accounts for an estimated 30%-40% of adult congenital cardiac abnormalities[5-7]. Ostium secundum defects are the most commonly reported ASD as compared to defects associated with the septum primum, sinus venosus, or unroofed coronary sinus which occur in descending frequency respectively[8]. Although surgical closure of ASD is considered to be safe, efficacious, and time-tested, it requires open heart surgery, longer hospital stays, and may not be suitable for elderly patients with concomitant comorbidities[9].
Morphology and clinical features of ASDs
Location, Morphology, and suitability for surgery vs. transcatheter intervention
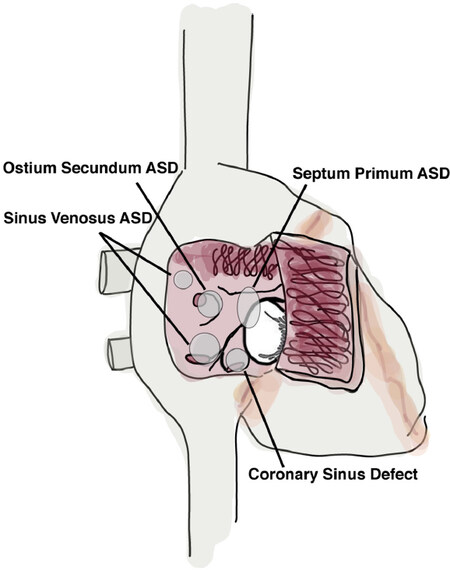
It is important to note that morphological variations of different types of ASDs, which determines whether a particular defect is amenable for transcatheter closure. Briefly, ASDs fit into four major classes: ostium secundum, ostium primum, sinus venosus, and unroofed coronary sinus [Figure 1]. Ostium secundum defects are characterized by enlarged foramen ovale with insufficient septem secondum development, causing incomplete closure and fusion of the atrial septum. Secundum type defects are the most common atrial septal malformation accounting for up to 80% of ASDs[10]. Secundum type defects are considered ideal for transcatheter ASD closure due to their size and surrounding tissue for device fixture. Ostium primum defects, also known as endocardial cushion defects, are defects at the level left or right atrioventricular valves. Sinus venosus defects are located in proximity to either the superior or inferior vena cava. Lastly, coronary sinus septal defects are characterized as an “unroofing” of the coronary sinus in which allows communication between the coronary sinus and the LA. Mixed defects, or those involving multiple defect types are also possible, though less commonly reported are also typically repaired with open surgery. Although, surgical repair is considered as the standard method of treatment for all but secundum type defects, case reports are exist describing multiple ASDs and coronary sinus defects which transcatheter closure was successful without significant valvular impairment or conduction disturbance[11,12].
ASD variation with age
Clinical characteristics of ASDs differ significantly in pediatric populations as compared to adults. ASDs are detected in asymptomatic children with increasing frequency due to non-invasive screening modalities such as echocardiography, routine ECG, and even prior to birth during routine obstetric wellness sonograms[13]. Furthermore, studies show referral to specialty services occurs at an earlier age if subtle findings are detected on physical examination despite lack of symptoms. One such single center retrospective study indicated the median age of diagnosis was 5 months of age[14]. Areas of increasing interest in pediatric management include predictors of spontaneous ASD closure. Many predictors of spontaneous closure have been proposed; the longest held predictor appears to be size of the ASD at time of detection with small defects (3-5 mm) having up to 87% closure rates and large defects (> 8 mm) conferring much lower closure rates (0%-8%)[14-16]. Others suggest the use of patient age at time of detection or normal weight gain after detection as clinical predictors for spontaneous ASD closure[17].
Adult populations with ASDs are typically asymptomatic with great variability in the onset of symptoms. More common symptoms appear to be early onset atrial flutter or atrial fibrillation due to atrial stretch, and less commonly decompensated right heart failure in patients under 40 years of age[18]. The natural history and subsequent prognosis were reported by Campbell[19] with progressive worsening mortality approaching 90% by the 6th decade in patients with uncorrected defects.
Untreated atrial septal defect in the elderly
Elderly patients with hemodynamically significant defects more frequently encounter complications with long-term adverse consequences such atrial arrhythmia, pulmonary hypertension, and atrioventricular valvular insufficiencies related to chronic ventricular volume overload[20]. These patients have comparatively higher prevalence of co-morbid diseases including diabetes mellitus, stroke, systemic hypertension, chronic lung diseases atherosclerosis and coronary heart diseases[21-23]. Longstanding left to right shunt at the atrial level further results in a progressive atrial stretch and right ventricular dilatation which in turn eventually leads to tricuspid insufficiency[24]. The left heart may also be influenced by way of increased atrial pressure, chronic volume under load, and co-morbid diseases like systemic hypertension or coronary heart disease[6]. Furthermore, chronic LV unloading due to the left to right shunt, and diastolic compression of left ventricle by the dilated right ventricle may further reduce the LV end-diastolic volume in the chronic state. This so called “masked LV restriction” may lead to development of pulmonary edema secondary to LV dysfunction and left atrium (LA) pressure increase after ASD closure[22,25]. Due to the chronic nature of the condition, patients usually adjust their activity level to adapt to their relative disabilities, and invasive interventions are placed under increasing scrutiny due to the paucity of evidence for survival benefit. Prospective studies evaluating quality of life improvements, or elucidating risk vs. objective benefit are called for to establish the role of ASD closure in the elderly.
Imaging modalities for ASD evaluation
Echocardiography
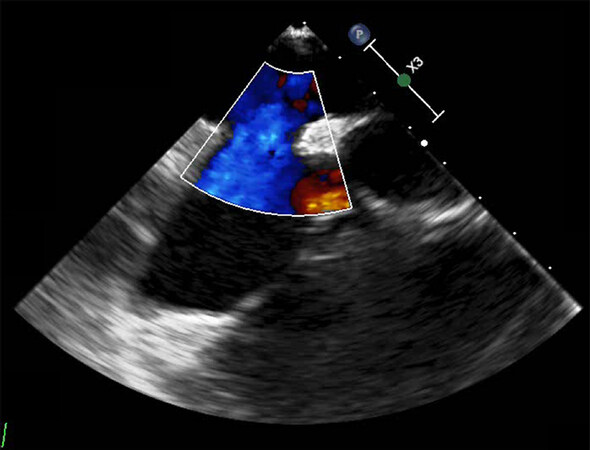
Conventional transthoracic echocardiography (TTE) is capable of identifying the presence of ASDs, characterizing chamber dilatation, estimated pulmonary artery pressure, shunt ratio, and other coexisting cardiac conditions. Figure 2 demonstrates doppler imaging of an unrepaired ASD. Tissue doppler imaging may be of particular use in elderly patients who suffer pronounced LV diastolic dysfunction. One recent study suggests patients at risk for post ASD closure congestive heart failure by measuring early mitral annular velocity to help direct volume management during and after ASD closure[26]. In regard to assessment of ASD morphology, including maximum defect dimensions and characterization of the surrounding tissue rim, 2D TTE is somewhat limited. These limitations are surmounted with the adjunct of transesophageal echocardiography (TEE) which offers a stepwise enhancement in characterizing the size, location, and tissue rim surrounding ASDs to determine suitability for transcatheter repair. TEE is considered a semi-invasive procedure so is undertaken only after initial evaluation with TTE[27,28].
3D echocardiography
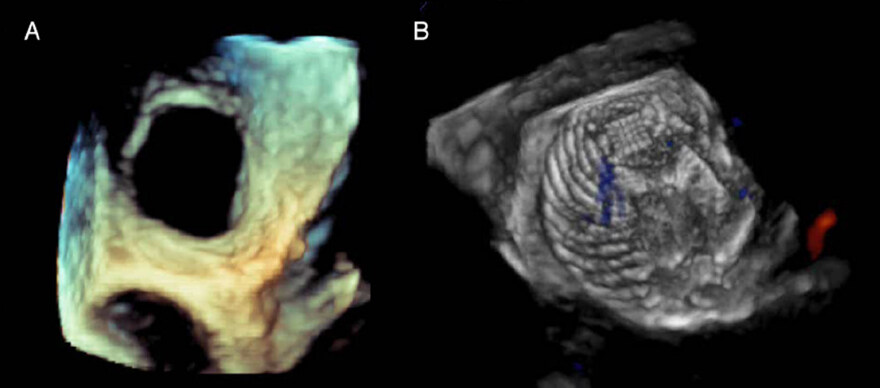
3D echocardiography provides better spatial visualization than conventional echocardiography. An example of a diagnostic 3D TEE visualizing an unrepaired defect can be seen in Figure 3A. 3D TEE can also depict 3D structures in great detail with high-resolution images allowing for enhanced understanding of complex valvular and congenital heart defects[27]. Initially, 3D echocardiography was reconstructed from serial 2D images, which is time-consuming and resource intensive. Nowadays real-time 3D echocardiography with matrix array transducer is available in TTE as well as TEE. 3D TTE is a promising technology capable of providing comprehensive en face images of ASD with the added benefits of being noninvasive, lower cost than TEE, portability, and carries better accessibility than TEE. 3D TTE has a potential to provide accurate information on ASD morphology such as size, location, and surrounding rims for treatment both in children and adult populations. Furthermore, real-time 3D TEE has utility in providing accurate real-time information about complex ASDs, especially those in patients with multiple ASDs, and allowing for real-time feedback of device deployment positioning[29,30].
Intrauterine Sonographic Detection of ASD
The International Society of Ultrasound in Obstetrics and Gynecology recently published guidelines on the detection of fetal cardiac anomalies in 2017 with the goal of improving early detection by obstetricians and family practitioners[31]. The feasibility of ASD detection via intrauterine sonogram has been demonstrated by many isolated case reports, retrospective analysis, and prospective studies[32-34]. Despite this, there are few reports on the sensitivity of intrauterine ASD detection (30%-74%), and should be relegated to pregnancies that carry high risk for cardiac abnormalities[34-36].
Transcranial doppler ultrasonography
Transcranial doppler ultrasonography (TCD) is a viable alternative to TTE or TEE for screening and follow-up evaluation of ASD. It offers a relative degree of comfort over TEE, and offers sensitivities equivalent to TTE in terms of identifying right to left shunts, but cannot detect other associated defects that echocardiography can[37].
Intracardiac Echocardiogram
Intracardiac echocardiography (ICE) offers superior visualization of the septal morphology during transcatheter device deployment[38]. It is, as the name suggests, invasive and requires additional femoral vessel access for deployment. Real time 4D ICE also appears to be on the horizon with reports on its development and pilot study usage are now emerging[39].
Management of ASD
Surgical vs. Medical management of Secundum ASD
ASDs are considered for closure in symptomatic patients where a left to right shunt is present with evidence of right heart pressure overload (right atrial or ventricular enlargement), and pulmonary to systemic blood flow ratio (Qp:Qs) is greater than 1.5:1[40]. In addition to right heart overload and Qp:Qs > 1.5:1, in asymptomatic patients, those whose pulmonary artery pressure is less than 50 percent systemic arterial pressure, and pulmonary vascular resistance is greater than one third the peripheral vascular resistance, without exercise induced cyanosis, are recommended for ASD closure[40-42].
Of great interest to clinicians in the age of readily available transcatheter repair of secundum type ASDs is the decision to pursue transcatheter or open surgical repair. One such study at Mayo Clinic sought to evaluate outcomes of surgically managed ASD cases as compared to medical management alone with a follow-up interval of 27 to 32 years after the index surgery. Study findings demonstrated that the survival rate was 74% as compared to 85% for age sex matched medically managed controls. In cases where surgical intervention occurred below the age of 24 years, survival rate reported was the same as age matched controls. Independent predictors of long-term survival were age at the time of operation and main pulmonary artery systolic Pressure (PASP)[43]. The more recent study of the Danish population found that there was a significant reduction in the life expectancy and lower quality of life in patients with small ASDs that did not meet criteria for repair when compared to the general population[44]. In another study, surgical vs. medical management were compared prospectively in a randomized clinical trial of 473 patients over the age of 40 years with a median follow up period of 7.3 years. Overall mortality rate was not statistically different. However, there was a higher rate of recurrent pneumonia noted in the medical arm. There was indeed a trend higher complication rates such as CHF, sudden cardiac death, and overall mortality in the medical arm, but was ultimately not statistically significance[45].
More recently the 2018 ACC/AHA task force undertook a meta-analysis seeking to understand differences in outcomes of medical vs interventional management of secundum type ASDs. Their analysis found 11 studies that met criteria for inclusion, and in most instances found a protective effect with bearing on reduction of symptoms, functional capacity, and improvement of hemodynamic characteristics following either surgical or transcatheter intervention[46]. Interestingly, in the same analysis, there was either insufficient data to determine relative risk of death, or a weakly positive protective effect after intervention, from the included studies. Furthermore, a nationwide study of patients with corrected and uncorrected ASDs were compared to the general population with interesting results; their findings demonstrated a relative reduction in mortality for patients who underwent repair of ASDs. But whether repaired or not, patients with ASDs patients experienced a shorter lifespan when compared to the general public[47]. Taking the results of these, and similar studies, may help establish important expectations when discussing intervention; quality of life and reduction of symptoms, rather than preventing mortality, may lead discussions pertaining to goals of therapy.
Surgical vs. transcatheter intervention
At the time of this writing, only secundum type defects have transcatheter devices approved for intervention. Primum, Sinus Venosus, and Coronary sinus defects still carry the recommendation of open surgical intervention with only rare reports of transcatheter interventions published[48-51]. In the pediatric population there are similar long term outcomes between surgical vs. transcatheter intervention, however cost analysis demonstrates a better value in terms of overall cost as well as shorter length of stay for patients undergoing transcatheter repair[52]. In the adult population, mortality between transcatheter and surgical intervention are similar, but long-term reintervention rates appear to vary between the two. In a review of 718 procedures, Kotowycz et al.[53] report that long term reintervention rates for transcatheter repair are more common than compared with conventional surgical approach. Other studies report similar findings - but prospective randomized studies have not been undertaken to determine true differences. Furthermore, patients more likely to undergo transcatheter repair are often higher risk than patients deemed appropriate for surgical intervention potentially skewing attempts to study differences in outcomes.
Of growing interest is the prospect of treating ASDs associated with sinus venosus defects, which are traditionally treated with open surgical repair. Presently, there are only case reports describing transcatheter approaches. Two such case reports technical success with correcting partial anomalous pulmonary venous return of the right upper pulmonary vein by deploying a covered stent graft into the affected pulmonary vein[54,55]. Abdullah et al.[56] describe an approach that combines covered stent grafts and occlusion devices to correct sinus venosus defects successfully in four patients; of which two required re-intervention with an additional covered stent or PFO closure device, but all without significant complications at the 12-month follow-up point[56]. Others have reported success with the immediate release patch, which has been under investigation in translational animal studies as a potential alternative to metallic devices[57,58].
Transcatheter devices available today for closure of secundum ASDs
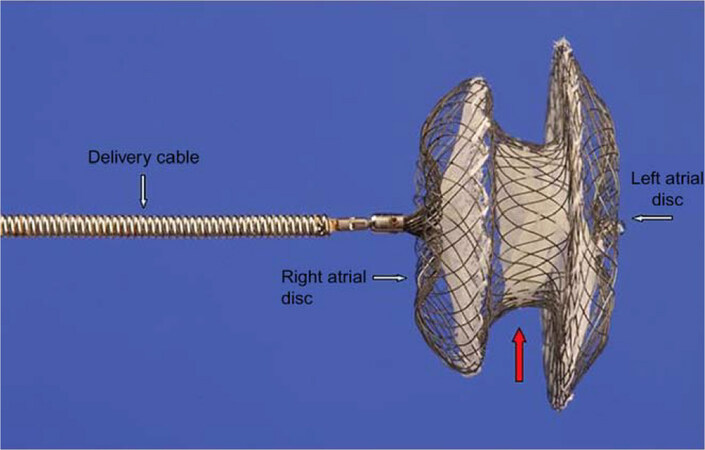
The origins of transcatheter ASD repair can be traced back to King’s report of non-operative ASD closure during cardiac catheterization in 1976[59]. Formal development of a device for ASD, however, is attributed to the Atrial Septal Defect Occluding System (ASDOS) submitted by Babic et al in 1990[60]. Though successive iterations made the device more user friendly and showed early promise, the ASDOS was abandoned in 2001 with the development of newer generations of transcatheter devices. A history of transcatheter device evolution has been detailed by Nassif et al.[61]. Today, transcatheter ASD closure is associated with low complications, short duration anesthesia, short hospital stay, and well documented long-term symptom follow up[62-64]. Transcatheter ASD closure is now considered the first choice of treatment as opposed to surgical intervention. The most widely employed device worldwide, and one of two FDA approved devices for use in North America is the Amplazer device, shown in Figure 4. Echocardiography, either ICE or TEE play a considerable role in the guidance of these procedures and in further assessment of the final results. Areas of active research focus on examining other imaging modalities like magnetic resonance imaging or computed tomography to construct 3D topographical visualizations of the heart and associated defects prior to transcatheter ASD closure[65-69]. A review of recent publications describing the outcomes and population size of the respective studies are listed in Table 1, including devices otherwise available outside of the United States.
Figure 4. St Jude Amplatzer Device, reproduced under creative commons license, Thomson and Quereshi 2015
Recent publications describing ASD device outcomes
| Device name | Manufacturer | Approval | Recent/ongoing studies | n | Significant findings |
|---|---|---|---|---|---|
| Cocoon | Vascular Concepts | CE Mark | Lairakdomrong et al.[70], 2013 - retrospective | 63 | 100% closure at 12 mo, 3 early embolization requiring surgical |
| Pakkret, Thailand | Thanopoulos et al.[71], 2014 - prospective observational | 92 | 100% closure at 6 mo, no adverse events | ||
| Ultrasept II | Cardia | CE Mark | Mijangos-Vázquez et al.[72], 2018 - retrospective | 30 | 100% closure at 6 mo, no adverse events |
| Eagan, MN, USA | Bartel et al.[73], 2010 - case series | 2 | 2 reports of fabric erosion requiring surgical removal | ||
| Aubry et al.[74], 2014 - case series | 9 | 2 out of 9 experienced fabric erosion requiring surgical removal | |||
| Bozyel and Özcan[75] - 2017, retrospective | 9 | 3 out of 9 patients with device required surgical removal | |||
| Chamié et al.[76], 2016 - case series | 4 | 4 out of 70 developed early fabric erosion, treated with device in device | |||
| Nit Occlude ASD-R | PFM Medical Mepro | CE Mark | Peirone et al.[77], 2014 - prospective observational | 73 | 98.6% closure at 11 mo, no adverse events |
| Köln, Germany | Bulut et al.[78], 2016 - prospective observational | 30 | 98% closure at 10 mo, 1 erosion requiring surgical removal | ||
| Ceraflex ASD | CE Mark | Astarcioglu et al.[79], 2015 - prospective non-randomized | 58 | 100% closure at 6 mo, no adverse events | |
| Apostolopoulou et al.[80], 2018 - retrospective | 183 | 100% closure at 22 mo, no adverse events | |||
| Figulla Flex II | Occlutech | CE Mark | Kenny et al.[81], 2018 - prospective randomized | 107 | 94.4% closure at 6 mo, 1 device embolization |
| Jena, Germany | Haas et al.[82], 2016 - retrospective | 1315 | 97.3% closure at 12 mo, 5 device embolization, 3 AV block | ||
| Godart et al.[83], 2014 - retrospective | 31 | 90.3% closure at 36 mo, 1 device embolization, 1 AV block | |||
| Roymanee et al.[84], 2015 - retrospective | 77 | 97.4% closure at 43 year, 2 device embolization, non-inferiority to ASO | |||
| Aytemir et al.[62], 2013 - retrospective | 58 | 99.3% closure at 12 mo, 2 device embolization, 4 embolic events, 2 device thrombosis | |||
| Kim et al.[85], 2019 - retrospective | 152 | 100% closure at 25 mo | |||
| Cardioform | WL Gore | CE Mark | GORE Assured Study, ongoing[86] | 522 | Clinical Trial NCT02985684, enrollment complete, final results by 2022 |
| Flagstaff, AZ, USA | FDA PMA | Hemptinne et al.[87], 2017 - retrospective | 26 | 100% closure at 6 mo, 5 wire frame fractures | |
| Kim et al.[85], 2019 - retrospective | 17 | 100% closure at 23 mo | |||
| Grohmann[88] - 2016, retrospective | 173 | 95.4% closure at 20 mo, 4 device embolization, 3 AV Block | |||
| Amplatzer | St. Jude Medical | CE Mark | Turner et al.[89], 2017 - prospective | 1000 | 97.9% closure at 24 mo, 1 embolization, 3 cardiac erosion |
| St. Paul, MN, USA | FDA | Spies et al.[90], 2007 - retrospective | 170 | 100% closure at 12 mo, 4 embolization, 1 TIA, | |
| Tomar et al.[91], 2011 - retrospective | 529 | 100% closure at 56 mo, 96.7% symptom free, 1 stroke | |||
| Kim et al.[85], 2019 - retrospective | 98 | 100% closure at 29 mo, 1 embolization | |||
| Post Market Surveilence (ASO 522)[92] | 602 | Clinical Trial NCT02353351, study terminated, results not published yet |
ASD characteristics amenable for percutaneous closure
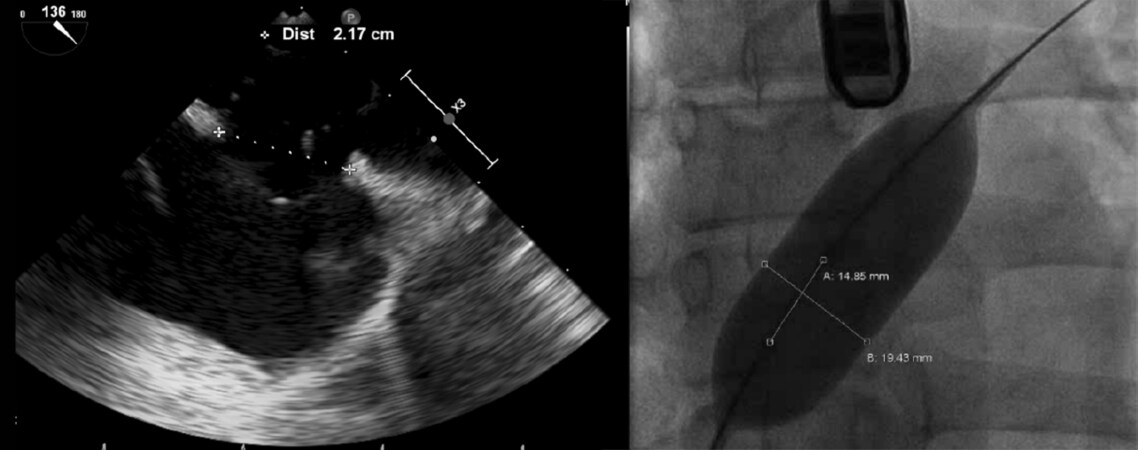
Two crucial parameters should be evaluated in patients with secundum septal defect prior to intervention: maximal ASD and surrounding rim dimensions. Presently, the Amplatzer device is capable of closing defects with a maximum defect diameter less than 38 mm[93]. An example of balloon sizing as assessed intraoperatively with balloon sizing as compared to real time TEE sizing can be seen in Figure 5. ASDs typically give the appearance of with ellipsoidal geometry that varies throughout the cardiac cycle[94,95]. Selection of optimal device size, particularly in patients undergoing the procedure without balloon sizing or multiple defects, involves measuring the major axis diameter of the defect during of ventricular end systole. More recently, real time 3D TEE is challenging the need for balloon sizing with stop-flow technique as an adjunct to prevent underestimation of defect size or tissue rims[96,97].
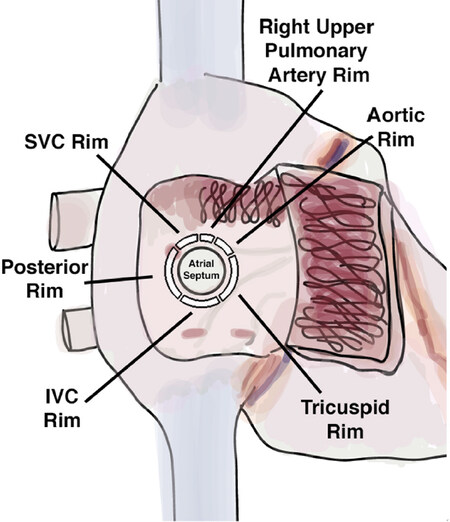
Transcatheter closure of ASDs with a maximal native diameter > 30 mm can be quiet challenging, and alternative techniques for deployment may be required, which will be discussed later. In regard to classification of surrounding rims, although there are some differences noted among studies, distances from ASD to aorta, superior vena cava, right upper pulmonary vein, inferior vena cava, coronary sinus, and atrioventricular valve are evaluated. Adequate tissue rim is defined by at least 5mm from the defect edge to the surrounding structures so as not to impinge on the vena cava, pulmonary vein, coronary sinus, tricuspid or mitral valve[28]. Figure 6 depicts areas of interest in measuring surrounding tissue rim dimensions.
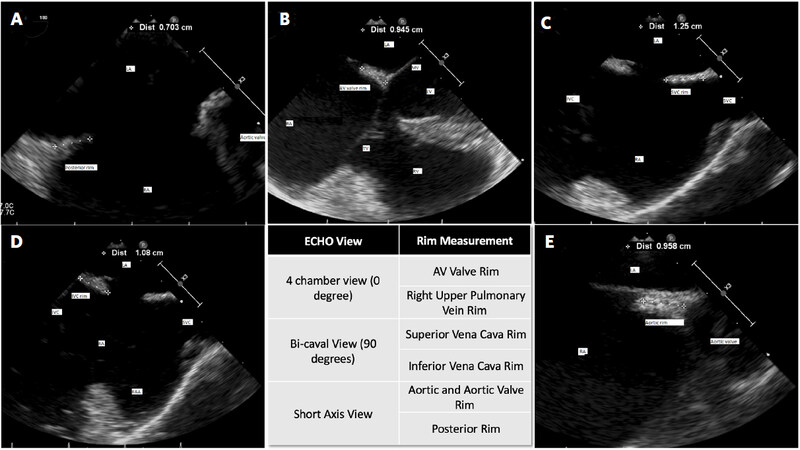
Figure 7 illustrates the tissue rim measurements as seen via intraoperative TEE. Tissue measurements are best taken as follows: AV valve and right upper pulmonary vein tissue rim are best viewed in the 4 chamber view, SVC and IVC rims are best measured in the Bi-Caval view, and the Aortic and posterior rim measurments are best taken in the short axis view. These are recommendations, but individual body habitus and variations in heart orientation may necessitate obtaining alternate views to accurately measure tissue rims. Interestingly, Yan et al.[98] describe generating a custom 3D model to visualize and assess device closure feasibility based on 3D TEE end systolic dimensions with 29 of 30 patients found to have deficient posterior-inferior rim size (< 3 mm), providing a proof of concept for simulated in-vivo device fitment prior to undergoing transcatheter intervention[98]. Though, caution should be maintained regarding attempting transcatheter closure with inadequate rim size, as many studies demonstrate increased risk for device embolization with difficult retrieval or conversion to open surgery[99-102].
Special issues in the management of elderly patients with ASD
Comparative benefits from ASD closure in the elderly population have historically been underreported as compared younger populations. The paradigm of non-operative management of previous generations had, in some ways, stymied broad acceptance and given cause to thwart intervention where there was no perceived benefit. However, percutaneous management of ASD in elderly patients has gained reluctant enthusiasm, as evidenced by analyzing trends in hospitalizations captured by the National Inpatient Sample Database[103-106]. The promise of shorter hospitalization time and reduced complication rates is tempered with the many difficulties faced perioperatively due to the tendency toward combined comorbidities. Realistic benefits of ASD closure include symptomatic relief, improvements of functional status as well as the overall improvement in the quality of life[25].
Outcomes of ASD closure in elderly patients
In two separate studies Swan and Khan both found that following ASD intervention, a small cohort of geriatric patients with a median age of 70 years old, saw improvement in their New York Heart Association (NYHA) class, 6 minute walk time and improvement in overall physical/mental health score in addition to an extremely high procedural success rate (98%)[23,107]. Furthermore, Nakagawa et al.[103] reported that after intervention in a population composed of patients 70 years or older with hemodynamically significant ASD, percutaneous closure is efficacious and safe. The intervention led to a significant improvement of PA pressure and NYHA functional class, as well as reversal of RV enlargement[103].
Similarly, in 2014 Komar et al.[108] studied the mid-term outcome of patients over the age of 60. Interestingly, their primary outcome was focused more on quality of life indices and functional benefits rather than complications or long term survival. Metrics such as time of sustained exercise before feeling short of breath, VO2max, and the SF-36 quality of life questionnaire to gauge the benefits of ASD closure. Symptomatic parameters like incidence of shortness of breath or time of exercise before shortness of breath both improved significantly; furthermore 88% of patients surveyed had a significant subjective improvement in quality of life 12 months following their index surgery[108].
Obstacles in transcatheter atrial septal defect closure in elderly patients
The most salient issue in elderly cases is not their primary pathology, but their co-morbid systemic and cardiac diseases. This necessitates careful preoperative evaluation of the associated risk factors as an essential aspect of successful treatment. Approximately one third of the patients showed systemic hypertension and systemic diseases like diabetes mellitus, and a considerable extent of pulmonary and neurological disease conditions were also present[109]. Among the cardiac co-morbidities pulmonary hypertension is reported in nearly 50 % of the cases, chronic atrial arrhythmia in more than 20% and ischemic heart disease in about 15% of the patients[110,111]. Post-closure pulmonary edema developed because of “masked LV restriction” may appear in 2% to 4% of the elderly cases may be evaluated with a balloon occlusion prior to ASD closure[112].
Similarly, diastolic dysfunction and stiffening of the LV causes increased left to right shunting, which may explain in part why the late diagnosis is established in elderly patients who were previously asymptomatic. Careful assessment of left ventricular and left atrial pressures via left heart catheterization during defect balloon occlusion and weighing potential hemodynamic consequences vs. perceived benefits of intervention, are especially important in the elderly patient population. Miranda et al report that left ventricular end diastolic pressure may help predict left atrial pressures in those undergoing ASD repair. They found that the vast majority of patients who had a baseline left ventricular end diastolic pressure > 15 mmHg developed significantly elevated left atrial pressure during balloon occlusion of ASD[113].
ASD and pulmonary arterial hypertension
Due to chronic right ventricular volume overload, elderly patients with hemodynamically significant ASDs have a tendency to present with pulmonary hypertension. Pulmonary hypertension develops as a result of increased pulmonary blood flow due to left-to-right shunting. However, the anomalous rise in pulmonary blood flow creates secondary physiologic changes such as pulmonary vascular intimal proliferation and medial hypertrophy that affect pulmonary vascular resistance[114,115]. The consequence of such changes has been observed to be reversible in younger patients, but may not be fully reversible in the elderly[116]. It is well understood that the natural course of ASD and the associated effect on pulmonary hypertension is notably worse than in patients without pulmonary hypertension[117]. Thus, pulmonary hypertension is traditionally considered an absolute contraindication to ASD intervention, especially surgical closure[118]. The expansion of therapeutic options for treating pulmonary hypertension may offer new avenues for ASD closure. An area of active research is the role of ASD closure in combination with new pulmonary hypertension treatments such as prostanoids, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors, even if initial hemodynamic parameters are unamenable to ASD closure[119-122]. More recent studies such as the North American Atrial Septal Defect Pulmonary Hypertension (NAAPH) Study demonstrate feasibility of ASD closure in patients with PAH with an aggressive “treat to repair” strategy which first addresses underlying pulmonary hypertension[123]. Optimization of elderly patients with concomitant pulmonary hypertension prior to ASD closure remains an area of active research.
Cardiac erosion after percutaneous ASD intervention
In patients with superoanterior rim deficiency, the increased risk of serious complication, i.e., “cardiac erosion” may increase after implantation of the device. The exact mechanism of “cardiac erosion” is not been well understood; previous clinical experience proposed that an aortic rim deficiency and oversized occlusion device may be highly correlated with cardiac erosion[124]. In response, updated instructions-to-user were published for the Amplatzer device with specific guidance for aorto-superior rim size specifications[125]. One recent case series reported that absence of the aortic rim was common finding among patients who developed erosion[126]. Subsequently, other putative risk factors were also reported as physicians modified their practices and over sizing became less common[127]. Specifically, deficient aortic or SVC rim size, along with balloon sizing were associated with increased risk of erosion[128]. It should be noted, however, that these studies are retrospective in nature, and prospective studies have not yet been undertaken to determine true causal relationships for erosion relating to rim size.
Future direction of transcatheter intervention for ASD
The transcatheter ASD repair has evolved from employment in select patients unable to undergo open surgical repair, to applications in pediatric populations, and is now gaining traction in the elderly. Where currently secundum type ASDs and limited case-reports of closure in other varients of ASD are now being reported, we may expect future devices to address these limitations. On the other hand, complications arising from this procedure, especially cardiac erosion, are still being reported. Progress over the last several decades in terms of safety and efficacy are impressive and point to a bright future in the treatment of congenital heart defects. We conclude this review by looking to the near and long-term future in the state of the field.
New devices for difficult ASD closure
Several technical modifications have been introduced over the years to address difficult transcatheter ASD closure, including delivery sheath modification, position deployment, or additional material to hold the left atrial disk inside the LA. Some advocate deployment with balloon assisted placement[129]. This technique, however, may cause injury to the pulmonary vein. The development of steerable catheters may offer improved techniques in positioning ASD devices[130]. Use of such a steerable catheter has been described in case reports, but has not yet been implemented in commercially available devices, offering an opportunity for future development[131].
Endovascular retrieval of embolized devices
A well described early and mid-term complication of transcatheter ASD closure is device dislodgement and embolization. The rote response, if the device has been fully deployed, is to convert to open surgery for retrieval and repair. Improving techniques for endovascular retrieval are supported by case reports, case series, and retrospective reviews of experience[132-134]. Common embolization sites are the left ventricle, abdominal aorta and femoral vessels[135,136]. Lastly, Martins, Mendez, and Anjos provide an excellent pictorial stepwise description of various retrieval techniques and devices, and even include demonstrative videos[137]. Protective devices to prevent embolization during surgery may be an area of future interest to prevent distal embolization periprocedurally[138].
Salvage of residual shunt with device-in-device intervention
Intracardiac devices that are malfunctioning, whether dislodged, malpositioned, or sub-optimally effective, are typically treated with open heart surgery for removal and remedy. At the present, there are only case reports describing “device-in-device” salvage to return function to such malfunctioning devices[75,139]. The concept of device-in-device salvage involves deploying a second device through residual defects the first device did not completely close, in order to provide an adequate seal zone the first device did not provide. These early reports may eventually become the foundational principals for guidelines to prevent conversion to open surgery, but no definitive conclusions can be drawn with such limited data. Likewise, residual shunts following surgical repair of ASDs may be of great interest in the case of secundum ASDs.
Gene thrapy, tissue engineering, and stem cell therapy for ASD
The goals of treatment in congenital cardiac malformations are ever shifting. Seventy-five years ago, researchers and clinicians sought to find appropriate screening criteria where risk factors for ASDs were poorly understood. With the advent of better screening methods and guidelines, the difficult decision of who should undergo surgery or medical management then became the diagnostic dilemma. With newer, safer, conventional and endovascular procedures well established, the next logical progression in the field is primary prevention of the disease process. Several reports proposing genes associated with ASD that may inform progress toward potential targets for gene therapy in genetically linked variants of ASD[140-142]. Furthermore, elucidating the temporal and spatial relationships among terminally differentiating cells during cardiogenesis may provide further insight into the precise moment in congenital defects begin[143,144].
Until primary prevention with gene therapy is technically feasible, other interventions may be on the horizon. Tissue compatibility of ASD closure devices remains an area of interest for researchers and clinicians alike. Biocompatible and bioabsorbable based devices are currently under investigation[145]. Those at the beginning of their surgical career may see 3D printed custom devices or bio inspired devices in the form of surgical glue or gels that can be deployed endovascularly emerge within their career[54,146-148].
Declarations
AcknowledgmentsMarguerite Zimmermann, MSN was responsible for creating the artwork contained in this article.
Authors’ contributionsMade substantial contributions with initial draft, subsequent revisions, and approved final draft: Zimmermann E, Hussain H, Avgerinos D
Made substantial contributions with subsequent revisions and approval of final draft:
Worku B, Dougenis D
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2019.
REFERENCES
2. Rostad H, Sørland SJ. Atrial septal defects of secundum type in patients less than 40 years of age: a follow-up study. Acta Med Scand Suppl 1981;209:29-35.
3. Shaalan A, Elrakhawy H, Alassal M, Wakeel E. Improvement after surgical closure of secundum atrial septal defects in adults. J Clin Exp Cardiolog 2017;8:2.
4. Post M. Association between pulmonary hypertension and an atrial septal defect. Neth Heart J 2013;21:331-2.
5. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900.
6. Kim NK, Park SJ, Choi JY. Transcatheter closure of atrial septal defect: does age matter? Korean Circ J 2011;41:633-8.
7. Kaplan S. Congenital heart disease in adolescents and adults. Natural and postoperative history across age groups. Cardiol Clin 1993;11:543-56.
8. Martin SS, Shapiro EP, Mukherjee M. Atrial septal defects-clinical manifestations, Echo assessment, and intervention. Clin Med Insights Cardiol 2015;8:93-8.
9. Dehghani H, Boyle AJ. Percutaneous device closure of secundum atrial septal defect in older adults. Am J Cardiovasc Dis 2012;2:133-42.
10. Eadie LA, King MW. Biotextiles for atrial septal defect repair. In: King MW, Gupta BS, Guidoin R, editors. Biotextiles as Medical Implants. Woodhead Publishing; 2013. pp. 536-62.
11. Cho MJ, Song J, Kim SJ, Choi EY, Lee SY, et al. Transcatheter closure of multiple atrial septal defects with the amplatzer device. Korean Circ J 2011;41:549-51.
12. Kijima Y, Taniguchi M, Akagi T. Catheter closure of coronary sinus atrial septal defect using Amplatzer Septal Occluder. Cardiol Young 2012;22:223-6.
13. van Velzen CL, Clur SA, Rijlaarsdam ME, Bax CJ, Pajkrt E, et al. Prenatal detection of congenital heart disease--results of a national screening programme. BJOG 2016;123:400-7.
14. Hanslik A, Pospisil U, Salzer-Muhar U, Greber-Platzer S, Male C. Predictors of spontaneous closure of isolated secundum atrial septal defect in children: a longitudinal study. Pediatrics 2006;118:1560-5.
15. Radzik D, Davignon A, van Doesburg N, Fournier A, Marchand T, et al. Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J Am Coll Cardiol 1993;22:851-3.
16. McMahon C, Feltes T, Fraley J, Bricker J, Grifka R, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart 2002;87:256-9.
17. Lin KM, Liang CD, Chien SJ, Lin YJ, Lin IC, et al. Predictors for Regression of Large Secundum Atrial Septal Defects Diagnosed in Infancy. Acta Cardiol Sin 2013;29:82-7.
18. Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006;114:1645-53.
20. Le Gloan L, Legendre A, Iserin L, Ladouceur M. Pathophysiology and natural history of atrial septal defect. J Thorac Dis 2018;10:S2854-63.
21. Spies C, Hijazi ZM. Transcatheter closure of secundum atrial septal defects in the elderly. Korean Circ J 2009;39:47.
22. Schubert S, Peters B, Abdul-Khaliq H, Nagdyman N, Lange PE, et al. Left ventricular conditioning in the elderly patient to prevent congestive heart failure after transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv 2005;64:333-7.
23. Swan L, Varma C, Yip J, Warr M, Webb G, et al. Transcatheter device closure of atrial septal defects in the elderly: technical considerations and short-term outcomes. Int J Cardiol 2006;107:207-10.
24. Beitzke D, Wolf F, Edelhauser G, Lammer J, Loewe C. Right heart dilatation in adult congenital heart disease: imaging appearance on cardiac magnetic resonance. Br J Radiol 2011;84:188-93.
25. Elshershari H, Cao Q, Hijazi Z. Transcatheter device closure of atrial septal defects in patients older than 60 years of age: immediate and follow-up results. J Invasive Cardiol 2008;20:173-6.
26. Takaya Y, Akagi T, Kijima Y, Nakagawa K, Watanabe N, et al. Echocardiographic estimates of left ventricular diastolic dysfunction do not predict the clinical course in elderly patients undergoing transcatheter atrial septal defect closure: impact of early diastolic mitral annular velocity. J Interv Cardiol 2017;30:79-84.
27. Taniguchi M, Akagi T, Watanabe N, Okamoto Y, Nakagawa K, et al. Application of real-time three-dimensional transesophageal echocardiography using a matrix array probe for transcatheter closure of atrial septal defect. J Am Soc Echocardiogr 2009;22:1114-20.
28. Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the american society of echocardiography and society for cardiac angiography and interventions. J Am Soc Echocardiogr 2015;28:910-58.
29. Johri AM, Witzke C, Solis J, Palacios IF, Inglessis I, et al. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr 2011;24:431-7.
30. Perk G, Lang RM, Garcia-Fernandez MA, Lodato J, Sugeng L, et al. Use of real time three-dimensional transesophageal echocardiography in intracardiac catheter based interventions. J Am Soc Echocardiogr 2009;22:865-82.
31. Bishop KC, Kuller JA, Boyd BK, Rhee EH, Miller S, et al. Ultrasound examination of the fetal heart. Obstet Gynecol Surv 2017;72:54-61.
32. Cay N, Unal O, Ipek A, Ikiz SS, Balaban M, et al. Prenatal diagnosis of isolated ostium secundum defect by ultrasound in the second trimester. Am J Cardiol 2015;115:S135.
33. Persico N, Moratalla J, Lombardi C, Zidere V, Allan L, et al. Fetal echocardiography at 11-13 weeks by transabdominal high-frequency ultrasound. Ultrasound Obstet Gynecol 2011;37:296-301.
34. Erős FR, Beke A. Congenital fetal anomalies and the role of prenatal ultrasound. In: Tudorache S, editor. Congenital Anomalies-From the Embryo to the Neonate. IntechOpen; 2018.
35. Hafner E, Scholler J, Schuchter K, Sterniste W, Philipp K. Detection of fetal congenital heart disease in a low-risk population. Perinat Diagn 1998;18:808-15.
36. Rajiah P, Mak C, Dubinksy TJ, Dighe M. Ultrasound of fetal cardiac anomalies. AJR Am J Roentgenol 2011;197:W747-60.
37. Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005;112:1063-72.
38. Acheampong B, Johnson JN, Hagler DJ, Cabalka AK, Cetta F, et al. Intracardiac echocardiography-guided device closure of non-PFO/ASD shunts. Struct Heart 2017;2:69-74.
39. Wildes D, Lee W, Haider B, Cogan S, Sundaresan K, et al. 4-D ICE: A 2-D array transducer with integrated ASIC in a 10-Fr Catheter for Real-Time 3-D intracardiac echocardiography. IEEE Trans Ultrason Ferroelectr Freq Control 2016;63:2159-73.
40. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e81-192.
41. Silversides CK, Dore A, Poirier N, Taylor D, Harris L, et al. Canadian cardiovascular society 2009 consensus conference on the management of adults with congenital heart disease: shunt lesions. Can J Cardiol 2010;26:e70-9.
42. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57.
43. Murphy JG, Gersh BJ, McGoon MD, Mair DD, Porter C-bJ, et al. Long-term outcome after surgical repair of isolated atrial septal defect: follow-up at 27 to 32 years. N Engl J Med 1990;323:1645-50.
44. Udholm S, Nyboe C, Karunanithi Z, Christensen AI, Redington A, et al. Lifelong burden of small unrepaired atrial septal defect: results from the danish national patient registry. Int J Cardiol 2019;283:101-6.
45. Attie F, Rosas M, Granados N, Zabal C, Buendía A, et al. Surgical treatment for secundum atrial septal defects in patients > 40 years old: a randomized clinical trial. J Am Coll Cardiol 2001;38:2035-42.
46. Oster M, Bhatt AB, Zaragoza-Macias E, Dendukuri N, Marelli A. Interventional therapy versus medical therapy for secundum atrial septal defect: a systematic review (part 2) for the 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:1579-95.
47. Nyboe C, Karunanithi Z, Nielsen-Kudsk JE, Hjortdal VE. Long-term mortality in patients with atrial septal defect: a nationwide cohort-study. Eur Heart J 2017;39:993-8.
48. Baruteau A, Jones MI, Riahi M, Nieves Velasco Forte M, Byrne N, et al. Early experience of transcatheter correction of superior sinus venosus atrial septal defect with partial anomalous pulmonary venous drainage. Arch Cardiovasc Dis 2019;11:129.
49. Riahi M, Velasco Forte MN, Byrne N, Hermuzi A, Jones M, et al. Early experience of transcatheter correction of superior sinus venosus atrial septal defect with partial anomalous pulmonary venous drainage. EuroIntervention 2018;14:868-76.
50. Mohammad Nijres B, Kenny D, Kazmouz S, Hijazi ZM. Transcatheter closure of unroofed coronary sinus using covered stents in an adult with drainage of the coronary sinus to the right ventricle after supra-annular tricuspid valve replacement. Catheter Cardiovasc Interv 2017;90:1154-7.
51. Roy M, Nandi D, Chattopadhyay A, Bandhyopadhyay B, Nayak HK. Transcatheter closure of coronary sinus atrial septal defect by Amplatzer septal occluder device. J Indian Coll Cardiol 2017;7:163-5.
52. Ooi YK, Kelleman M, Ehrlich A, Glanville M, Porter A, et al. Transcatheter versus surgical closure of atrial septal defects in children: a value comparison. JACC Cardiovasc Interv 2016;9:79-86.
53. Kotowycz MA, Therrien J, Ionescu-Ittu R, Owens CG, Pilote L, et al. Long-term outcomes after surgical versus transcatheter closure of atrial septal defects in adults. JACC Cardiovasc Interv 2013;6:497-503.
54. Garg G, Tyagi H, Radha AS. Transcatheter closure of sinus venosus atrial septal defect with anomalous drainage of right upper pulmonary vein into superior vena cava--an innovative technique. Catheter Cardiovasc Interv 2014;84:473-7.
55. Thakkar AN, Chinnadurai P, Breinholt JP, Lin CH. Transcatheter closure of a sinus venosus atrial septal defect using 3D printing and image fusion guidance. Catheter Cardiovasc Interv 2018;92:353-7.
56. Abdullah HAM, Alsalkhi HA, Khalid KA. Transcatheter closure of sinus venosus atrial septal defect with anomalous pulmonary venous drainage: innovative technique with long-term follow-up. Catheter Cardiovasc Interv 2019; doi: 10.1002/ccd.28364.
57. Franke J, Bertog SC, Sideris EB, Hofmann I, Wunderlich N, et al. Transcatheter patch closure of an atrial septal defect of sinus venosus type using the immediate release patch. Catheter Cardiovasc Interv 2015;86:154-9.
58. Sideris B, Sideris E, Calachanis M, Papantoniou V, Moulopoulos S. The immediate release patch in the correction of experimental atrial septal defects. Catheter Cardiovasc Interv 2010;76:572-7.
59. King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976;235:2506-9.
60. Babic UU, Grujicic S, Djurisic Z, Vucinic M. Transcatheter closure of atrial septal defects. Lancet 1990;336:566-7.
61. Nassif M, Abdelghani M, Bouma BJ, Straver B, Blom NA, et al. Historical developments of atrial septal defect closure devices: what we learn from the past. Expert Rev Med Devices 2016;13:555-68.
62. Aytemir K, Oto A, Ozkutlu S, Canpolat U, Kaya EB, et al. Transcatheter interatrial septal defect closure in a large cohort: midterm follow-up results. Congenit Heart Dis 2013;8:418-27.
63. Wang JK, Chiu SN, Lin MT, Chen CA, Lu CW, et al. Mid-to-long-term follow-up results of transcatheter closure of atrial septal defect in patients older than 40 years. Heart vessels 2017;32:467-73.
64. Alnasser S, Lee D, Austin PC, Labos C, Osten M, et al. Long term outcomes among adults post transcatheter atrial septal defect closure: Systematic review and meta-analysis. Int J Cardiol 2018;270:126-32.
65. Nicolay S, Salgado RA, Shivalkar B, Van Herck PL, Vrints C, et al. CT imaging features of atrioventricular shunts: what the radiologist must know. Insights imaging 2016;7:119-29.
66. White HD, Halpern EJ, Savage MP. Imaging of adult atrial septal defects with CT angiography. JACC Cardiovasc Imaging 2013;6:1342-5.
67. Khoshpouri P, Khoshpouri P, Bedayat A, Ansari-Gilani K, Chalian H. Interatrial septum: A pictorial review of congenital and acquired pathologies and their management. Clin Imaging 2019;55:53-64.
68. Song J, Kim Y, Aigerim T. Usefulness of cardiac computed tomography in transcatheter closure of atrial septal defect with amplatzer septal occluder. Russ J of Cardiol 2013;47:35-9.
69. Chia RC, Salazar P, Al-Yafi M, Romano S, Farzaneh-Far A. Secundum Atrial Septal Defect. QJM 2018;111:571-2.
70. Lairakdomrong K, Srimahachota S, Lertsapcharoen P, Chaipromprasit J, Boonyaratavej S, et al. Clinical results of large secundum atrial septal defect closure in adult using percutaneous transcatheter Cocoon atrial septal occluder. J Med Assoc Thai 2013;96:1127-34.
71. Thanopoulos BD, Biasco L, Dardas P, De Backer O, Avraamides P, et al. Catheter closure of atrial septal defects using the Cocoon septal occluder: preliminary results of a European multicenter study. Int J Cardiol 2014;177:418-22.
72. Mijangos-Vázquez R, García-Montes AJ, Soto-López EM, Guarner-Lans V, Zabal C. Atrial septal defect closure with the new Cardia Ultrasept II™ device with interposed Goretex patch: Mexican experience-has the perforation of Ivalon’s membrane been solved? Cardiol Young 2018;28:709-14.
73. Bartel T, Bonaros N, Muller S. Device failure weeks to months after transcatheter closure of secundum type atrial septal defects. Heart 2010;96:1603.
74. Aubry P, Brochet E, du Fretay XH, Bouton-Brochet S, Ibrahim H, et al. Early malfunction of polyvinyl alcohol membrane-covered atrial septal defect closure devices. Circ Cardiovasc Interv 2014;7:721-2.
75. Bozyel S, Sahin T, Dervis E, Aktas M, Saskin H. A massive left-to-right shunt due to delayed spontaneous perforation of polyvinyl alcohol membrane of atrial septal occluder. Turk Kardiyol Dern Ars 2017;45:541-4.
76. Chamie F, Maia J, Giuliano LC. Device-in-device: a transcatheter alternative to surgical explantation of a failing atrial septal defect intracardiac prosthesis. Catheter Cardiovasc Interv 2016;88:239-43.
77. Peirone A, Contreras A, Ferrero A, da Costa RN, Pedra SF, et al. Immediate and short-term outcomes after percutaneous atrial septal defect closure using the new nit-occlud ASD-R device. Catheter Cardiovasc Interv 2014;84:464-70.
78. Bulut MO, Yucel IK, Kucuk M, Balli S, Basar EZ, et al. Initial experience with the Nit-Occlud ASD-R: short-term results. Pediatr Cardiol 2016;37:1258-65.
79. Astarcioglu MA, Kalcik M, Sen T, Aykan AC, Gokdeniz T, et al. Ceraflex versus Amplatzer occluder for secundum atrial septal defect closure. Multicenter clinical experience. Herz 2015;40 Suppl 2:146-50.
80. Apostolopoulou SC, Tsoutsinos A, Laskari C, Kiaffas M, Rammos S. Large single centre experience with the Cera and CeraFlex occluders for closure of interatrial communications: usefulness of the flexible rotation feature. Cardiovasc Interv Ther 2018;33:70-6.
81. Kenny D, Eicken A, Dahnert I, Boudjemline Y, Sievert H, et al. A randomized, controlled, multi-center trial of the efficacy and safety of the Occlutech Figulla Flex-II Occluder compared to the Amplatzer Septal Occluder for transcatheter closure of secundum atrial septal defects. Catheter Cardiovasc Interv 2019;93:316-21.
82. Haas NA, Soetemann DB, Ates I, Baspinar O, Ditkivskyy I, et al. Closure of secundum atrial septal defects by using the occlutech occluder devices in more than 1300 patients: the IRFACODE project: a retrospective case series. Catheter Cardiovasc Interv 2016;88:571-81.
83. Godart F, Houeijeh A, Recher M, Francart C, Polge AS, et al. Transcatheter closure of atrial septal defect with the Figulla® ASD Occluder: a comparative study with the Amplatzer® Septal Occluder. Arch Cardiovasc Dis 2015;108:57-63.
84. Roymanee S, Promphan W, Tonklang N, Wongwaitaweewong K. Comparison of the Occlutech® Figulla® septal occluder and Amplatzer® septal occluder for atrial septal defect device closure. Pediatr Cardiol 2015;36:935-41.
85. Kim AY, Jung SY, Chang JY, Jung JW, Choi JY. Early to mid-term follow-up outcomes of percutaneous closure of atrial septal defects using recent generation devices: a single-center experience. Korean Circ J 2019;49:326-35.
86. O’Byrne ML, Glatz AC, Gillespie MJ. Transcatheter device closure of atrial septal defects: more to think about than just closing the hole. Curr Opin Cardiol 2018;33:108-16.
87. de Hemptinne Q, Horlick EM, Osten MD, Millan X, Tadros VX, et al. Initial clinical experience with the GORE® CARDIOFORM ASD occluder for transcatheter atrial septal defect closure. Catheter Cardiovasc Interv 2017;90:495-503.
88. Grohmann J, Wildberg C, Zartner P, Abu-Tair T, Tarusinov G, et al. Multicenter midterm follow-up results using the gore septal occluder for atrial septal defect closure in pediatric patients. Catheter Cardiovasc Interv 2017;89:E226-32.
89. Turner DR, Owada CY, Sang CJ Jr, Khan M, Lim DS. Closure of secundum atrial septal defects with the AMPLATZER septal occluder: a prospective, multicenter, post-approval study. Circ Cardiovasc Interv 2017;10:e004212.
90. Spies C, Timmermanns I, Schräder R. Transcatheter closure of secundum atrial septal defects in adults with the Amplatzer septal occluder: intermediate and long-term results. Clin Res Cardiol 2007;96:340.
91. Tomar M, Khatri S, Radhakrishnan S, Shrivastava S. Intermediate and long-term followup of percutaneous device closure of fossa ovalis atrial septal defect by the Amplatzer septal occluder in a cohort of 529 patients. Ann Pediatr Cardiol 2011;4:22-7.
92. Villablanca PA, Briston DA, Rodés-Cabau J, Briceno DF, Rao G, et al. Treatment options for the closure of secundum atrial septal defects: a systematic review and meta-analysis. Int J Cardiol 2017;241:149-55.
93. Akagi T. Catheter intervention for adult patients with congenital heart disease. J Cardiol 2012;60:151-9.
94. Maeno YV. Dynamic morphology of the secundum atrial septal defect evaluated by three dimensional transoesophageal echocardiography. Heart 2000;83:673-7.
95. Acar P, Saliba Z, Bonhoeffer P, Aggoun Y, Bonnet D, et al. Influence of atrial septal defect anatomy in patient selection and assessment of closure with the Cardioseal device; a three-dimensional transoesophageal echocardiographic reconstruction. Eur Heart J 2000;21:573-81.
96. Yang MC, Wu JR. Recent review of transcatheter closure of atrial septal defect. Kaohsiung J Med Sci 2018;34:363-9.
97. Boon I, Vertongen K, Paelinck BP, Demulier L, Van Berendoncks A, et al. How to Size ASDs for Percutaneous Closure. Pediatr Cardiol 2018;39:168-75.
98. Yan C, Wang C, Pan X, Li S, Song H, et al. Three-dimensional printing assisted transcatheter closure of atrial septal defect with deficient posterior-inferior rim. Catheter Cardiovasc Interv 2018;92:1309-14.
99. Amedro P, Bayburt S, Assaidi A, Kreitmann B, Habib G, et al. Should transcatheter closure of atrial septal defects with inferior-posterior deficient rim still be attempted? J Thorac Dis 2019;11:708-16.
100. Levi DS, Moore JW. Embolization and retrieval of the Amplatzer septal occluder. Catheter Cardiovasc Interv 2004;61:543-7.
101. Rosenfeld HM, van der Velde ME, Sanders SP, Colan SD, Parness IA, et al. Echocardiographic predictors of candidacy for successful transcatheter atrial septal defect closure. Cathet Cardiovasc Diagn 1995;34:29-34.
102. Ramalingam R, Patil S, Setty N, Kharge J, Puttegowda B, et al. Tough, but not impossible-Retrieval of large atrial septal occluder devices embolized to left atrium. Interv Med Appl Sci 2017;9:42-6.
103. Nakagawa K, Akagi T, Taniguchi M, Kijima Y, Goto K, et al. Transcatheter closure of atrial septal defect in a geriatric population. Catheter Cardiovasc Interv 2012;80:84-90.
104. Agarwal S, Sud K, Menon V. Nationwide hospitalization trends in adult congenital heart disease across 2003-2012. J Am Heart Assoc 2016;5:e002330.
105. Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the US. J Am Coll Cardiol 2009;54:460-7.
106. Burchill LJ, Gao L, Kovacs AH, Opotowsky AR, Maxwell BG, et al. Hospitalization trends and health resource use for adult congenital heart disease-related heart failure. J Am Heart Assoc 2018;7:e008775.
107. Khan A, Tan J, Li W, Dimopoulos K, Spence M, et al. The impact of transcatheter atrial septal defect closure in the older population: a prospective study. JACC Cardiovasc Interv 2010;3:276-81.
108. Komar M, Przewlocki T, Olszowska M, Sobien B, Podolec P. The benefit of atrial septal defect closure in elderly patients. Clin Interv Aging 2014;9:1101.
109. Thilén M, Christersson C, Dellborg M, Mattsson E, Trzebiatowska-Krzynska A, et al. Catheter closure of atrial septal defect in the elderly (≥ 65 years). A worthwhile procedure. Int J Cardiol 2016;218:25-30.
110. Okamoto H. .
111. Faccini A, Butera G. Atrial septal defect (ASD) device trans-catheter closure: limitations. J Thorac Dis 2018;10:S2923-30.
112. Ewert P, Berger F, Nagdyman N, Kretschmar O, Dittrich S, et al. Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure? Catheter Cardiovasc Interv 2001;52:177-80.
113. Miranda WR, Hagler DJ, Reeder GS, Warnes CA, Connolly HM, et al. Temporary balloon occlusion of atrial septal defects in suspected or documented left ventricular diastolic dysfunction: Hemodynamic and clinical findings. Catheter Cardiovasc Interv 2019;93:1069-75.
114. Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958;18:533-47.
115. Heath D, Helmholz HF Jr, Burchell HB, Dushane JW, Kirklin JW, et al. Relation between structural change in the small pulmonary arteries and the immediate reversibility of pulmonary hypertension following closure of ventricular and atrial septal defects. Circulation 1958;18:1167-74.
116. Yong G, Khairy P, De Guise P, Dore A, Marcotte F, et al. Pulmonary arterial hypertension in patients with transcatheter closure of secundum atrial septal defects: a longitudinal study. Circ Cardiovasc Interv 2009;2:455-62.
117. Horer J, Eicken A, Muller S, Schreiber C, Cleuziou J, et al. Risk factors for prolonged intensive care treatment following atrial septal defect closure in adults. Int J Cardiol 2008;125:57-61.
118. Zwijnenburg R, Baggen V, Witsenburg M, Roos-Hesselink J, Van Den Bosch A. Prediction of pulmonary hypertension long-term after atrial septal defect closure. Eur Heart J 2017;38.
119. Kijima Y, Akagi T, Takaya Y, Akagi S, Nakagawa K, et al. Treat and repair strategy in patients with atrial septal defect and significant pulmonary arterial hypertension. Circulation 2015;80:227-34.
120. Kimura M, Tamura Y, Takei M, Yamamoto T, Ono T, et al. Dual phosphodiesterase type 5 inhibitor therapy for refractory pulmonary arterial hypertension: a pilot study. BMC Pulm Med 2015;15:62.
121. Li Q, Dimopoulos K, Zhang C, Zhu Y, Liu Q, et al. Acute effect of Inhaled Iloprost in children with pulmonary arterial hypertension associated with simple congenital heart defects. Pediatr Cardiol 2018;39:757-62.
122. Brida M, Gatzoulis MA. Pulmonary arterial hypertension in adult congenital heart disease. Heart 2018;104:1568-74.
123. Bradley EA, Chakinala M, Billadello JJ. Usefulness of medical therapy for pulmonary hypertension and delayed atrial septal defect closure. Am J Cardiol 2013;112:1471-6.
124. Jalal Z, Hascoet S, Baruteau AE, Iriart X, Kreitmann B, et al. Long-term complications after transcatheter atrial septal defect closure: a review of the medical literature. Can J Cardiol 2016;32:1315 e11-8.
125. Mallula K, Amin Z. Recent changes in instructions for use for the Amplatzer atrial septal defect occluder: how to incorporate these changes while using transesophageal echocardiography or intracardiac echocardiography? Pediatr Cardiol 2012;33:995-1000.
126. Amin Z. Echocardiographic predictors of cardiac erosion after Amplatzer septal occluder placement. Catheter Cardiovasc Interv 2014;83:84-92.
127. Jung SY, Choi JY. Transcatheter closure of atrial septal defect: principles and available devices. J Thorac Dis 2018;10:S2909-22.
128. McElhinney DB, Quartermain MD, Kenny D, Alboliras E, Amin Z. Relative risk factors for cardiac erosion following transcatheter closure of atrial septal defects: a case-control study. Circulation 2016;133:1738-46.
129. Narin N, Baykan A, Argun M, Ozyurt A, Pamukcu O, et al. New modified balloon-assisted technique to provide appropriate deployment in the closure of large secundum atrial septal defect using amplatzer septal occluder in children. J Invasive Cardiol 2014;26:597-602.
130. Al-Qbandi MH, Al-Bannai OA, Behbehani FQ. .
131. Yucel IK, Balli S, Kucuk M, Celebi A. Use of steerable delivery catheter to successfully deliver a Ceraflex septal occluder to close an atrial septal defect in a child with interrupted inferior vena cava with azygos continuation. Turk Kardiyol Dern Ars 2016;44:244-7.
132. Kudumula V, Stumper O, Noonan P, Mehta C, De Giovanni J, et al. Transcatheter retrieval of cardiovascular foreign bodies in children: a 15-year single centre experience. Pediatr Cardiol 2017;38:1183-90.
133. Herbert JT, Kertai MD. Transesophageal echocardiography use in diagnosis and management of embolized intravascular foreign bodies. Semin Cardiothorac Vasc Anesth 2018;22:100-3.
134. Shebani SO, Rehman R, Taliotis D, Magee A, Hayes NJ, et al. Techniques for transcatheter retrieval of the occlutech ASD device United Kingdom-European multicenter report. Catheter Cardiovasc Interv 2017;89:690-8.
135. Durmuş G, Kalyoncuoğlu M, Belen E, Can MM. Retrieval of Embolized Atrial Septal Defect Closure Device in the Left Iliac Artery. Am J Cardiol 2018;121:e156.
136. Crawford DA, Naidu SG, Shah AA, Davila VJ, Stone WM. Endovascular retrieval of an embolized atrial septal occluder device from the abdominal aorta. Vasc Endovascular Surg 2018;52:669-73.
137. Martins DS, Mendes IC, Silva JR, Anjos R. .
138. Pavithran S, Sivakumar K. A novel snare assistance safeguards against early embolization of devices and facilitates quick retrieval of malpositioned devices in atrial septal defects with deficient margins. Ann Pediatr Cardiol 2015;8:189-95.
139. Bhattacharyya S, Ilsley CD, Baltabaeva A. Disintegration of polyvinyl alcohol membrane covering atrial septal defect closure device. Eur Heart J Cardiovasc Imaging 2015;16:1153.
140. Teekakirikul P, Zhu W, Xu X, Smith AM, Wang C, et al. Identification of a novel sarcomeric TPM1 variant in familial atrial septal defect. Circulation 2018;138:A15276.
141. Su W, Wang RC, Lohano MK, Wang L, Zhu P, et al. Identification of two mutations in PCDHGA4 and SLFN14 genes in an atrial septal defect family. Curr Med Sci 2018;38:989-96.
142. Wang J, Luo J, Chen Q, Wang X, He J, et al. Identification of LBX2 as a novel causal gene of atrial septal defect. Int J cardiol 2018;265:188-94.
143. DeLaughter DM, Bick AG, Wakimoto H, McKean D, Gorham JM, et al. Single-cell resolution of temporal gene expression during heart development. Dev Cell 2016;39:480-90.
144. Bulatovic I, Mansson-Broberg A, Sylven C, Grinnemo KH. Human fetal cardiac progenitors: the role of stem cells and progenitors in the fetal and adult heart. Best Pract Res Clin Obstet Gynaecol 2016;31:58-68.
145. Hameed A, Duffy GP, Hasan B, Fatimi SH. Tissue engineered solutions for intracardiac septal defects: a large step forward in an unmet clinical need. Ann Surg 2017;265:e11-2.
146. Lang N, Pereira MJ, Lee Y, Friehs I, Vasilyev NV, et al. A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects. Sci Transl Med 2014;6:218ra6.
147. Tang B, Su F, Sun X, Wu Q, Xing Q, et al. Recent development of transcatheter closure of atrial septal defect and patent foramen ovale with occluders. J Biomed Mater Res B Appl Biomater 2018;106:433-43.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zimmermann E, Hussain H, Worku B, Dougenis D, Avgerinos D. Atrial septal defect repair in the age of transcatheter devices. Vessel Plus 2019;3:31. http://dx.doi.org/10.20517/2574-1209.2019.010
AMA Style
Zimmermann E, Hussain H, Worku B, Dougenis D, Avgerinos D. Atrial septal defect repair in the age of transcatheter devices. Vessel Plus. 2019; 3: 31. http://dx.doi.org/10.20517/2574-1209.2019.010
Chicago/Turabian Style
Zimmermann, Eric, Hafiz Hussain, Berhane Worku, Dimitrios Dougenis, Dimitrios Avgerinos. 2019. "Atrial septal defect repair in the age of transcatheter devices" Vessel Plus. 3: 31. http://dx.doi.org/10.20517/2574-1209.2019.010
ACS Style
Zimmermann, E.; Hussain H.; Worku B.; Dougenis D.; Avgerinos D. Atrial septal defect repair in the age of transcatheter devices. Vessel Plus. 2019, 3, 31. http://dx.doi.org/10.20517/2574-1209.2019.010
About This Article
Copyright
Data & Comments
Data

 Cite This Article 9 clicks
Cite This Article 9 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.