Clinical and perioperative applications of three-dimensional echocardiography
Abstract
The use of three-dimensional echocardiography, both in the clinical cardiology and perioperative settings, has increased thanks to its ability to add important information to the standard bi-dimensional exam and to evaluate structures without geometric assumptions. Both real time three dimensional (3D) transesophageal echo and offline quantitative measurements from 3D acquisitions have become integral for qualitative and quantitative analysis of structures and for surgical and procedural guidance. This review aims to provide an overview on the applications of 3D echo, with particular reference to the perioperative settings.
Keywords
Introduction
At the end of the 20th century, The New England Journal of Medicine released a landmark editorial listing the 11 most important medical achievements of the past 1000 years; body imaging was included as one of them[1]. Among all the imaging modalities developed over the decades, echocardiography is now one of the most widely used diagnostic test.
The history of echocardiography, sprinkled with many interesting events and anecdotes[2] cataloguing the journey towards currently used echo modalities, is quite intriguing. Standard mono- and bi-dimensional echocardiographic examination for transthoracic and transesophageal echocardiography allow the physician to obtain crucial information on cardiac structures, function, and hemodynamics in a remarkably quick and non-invasive or minimally invasive manner. In recent years, the never-ending quest for better quality and more detailed images, fueled by the invaluable contribution of the newest advances in hardware and software technologies, has led physicians to “usher in the third dimension”[3]. Three-dimensional echocardiography (3D echo) can potentially overcome many of the limitations intrinsic to mono- and bi-dimensional echocardiography. The great opportunities offered by this relatively novel technique have aroused the interest of many: In order to give a sense of it, a total of 9631 papers could be retrieved on Pubmed by searching “three dimensional echocardiography” (April, 2019).
This review aims to provide an overview on the applications of 3D echo, with particular reference to the perioperative settings.
Three-dimensional echocardiographic evaluation of cardiac structures
As a general principle, 3D echo is based on the acquisition of pyramidal datasets used to sample the cardiac structures of interest, acquired over a single beat [real-time (RT)] or several beats (multi-beat). Multi-beat acquisitions use ECG gating to “stitch” volume slices together by means of a dedicated software to generate a 3D image[3].
The accuracy and feasibility of 3D echo in studying valvular and congenital lesions has been largely demonstrated in the operating room, where 3D transesophageal echo (3D TEE) has been able to accurately predict valve morphology and provide complementary anatomic information to two-dimensional TEE (2D TEE)[4]. Unsurprisingly, 3D TEE has now been widely integrated into the workflow for guiding complex interventional procedures[5].
Adequate 2D image quality is an essential prerequisite for the evaluation of any cardiac structure by means of 3D echo. Moreover, inclusion of the entire cardiac structure of interest within the pyramidal dataset is of utmost importance. Of note, this can be quite challenging in patients with dilated ventricles (both left or right ventricle).
Once images are acquired, a key advantage of 3D over 2D echo is represented by the fact that measurements do not rely on any geometric assumptions. Dataset analysis is therefore not affected by foreshortened or off-axis views, as opposed to traditional 2D assessment.
However, the higher spatial resolution of 3D echo comes at the expense of lower temporal resolution, as compared to other echo modalities; while this might constitute a disadvantage (especially when assessing regional wall motion), careful technical adjustments of the images might still provide reasonable accuracy[6]. Of note, poor temporal resolution could be improved by a multi-beat acquisition that stitches volumes together. Notably, this modality requires a motionless patient (without interference of electrocautery, arrhythmias, or ventilation).
With the advent of newer, more powerful TEE and TTE probes, this issue has been significantly improved with adequate frame rates from a single beat acquisition.
Nevertheless, as elegantly summarized by Surkova and colleagues[7], the specific advantages of 3D echo over other imaging modalities - including its portability, absence of ionizing radiation, and the full compatibility with implanted cardiac devices like pacemakers and defibrillators- outweigh the aforementioned limitations in most cases.
Evaluation of the left ventricle
Subjective interpretation of left ventricular (LV) volumes and function has long been recognized as one of the greatest limitations of mono- and bi-dimensional echocardiography[8]. Accurate interpretation of LV volumes and function becomes even more difficult in the not uncommon case of patients with regional LV wall motion abnormalities, including aneurysmal LV for which conventional 2D echo has been demonstrated as not accurate in determining absolute LV volumes and ejection fraction (EF) because of LV asymmetry[9].
Validation of echo against cardiac magnetic resonance imaging (MRI) (regarded as the gold-standard), nuclear imaging, and computed tomography (CT) has consistently shown 3D echo to be more accurate and reproducible compared to conventional 2D evaluation[10]. In a meta-analysis including 23 studies (1,638 echocardiograms), Dorosz et al.[11] showed that 3D echo, as compared to cardiac MRI, systematically underestimates volumes and has wide limits of agreement; its performance, however, remains better than traditional 2D methods. Of note, greater magnitude of bias was reported in patients with poor quality data sets and/or large ventricles, likely due to the known difficulty to include larger structures within the 3D pyramidal dataset. Similar results have been obtained in other series[12], confirming the systematic underestimation of MRI-derived volumes by 3D echo.
These concepts have been incorporated into the latest edition of the American Society of Echocardiography (ASE) guidelines on chamber quantification, which now recommend different cutoffs to define normal LV 3D parameters[6].
A certain degree of underestimation by 3D echo has also been demonstrated with regard to LV mass assessment as compared to cardiac MRI, although improvements in terms of accuracy have been achieved more recently. In a meta-analysis including 25 studies comparing 3D echo vs. cardiac MRI, Shimada et al.[13] found that papers published in or before 2004 reported high heterogeneity (I2 = 69%) and significant underestimation of LV mass by 3D echo [-5.7 g, 95% confidence interval (95%CI) -11.3 to -0.2, P = 0.04], papers published from 2005 to 2007 were still heterogeneous (I2 = 60%) but showed less systematic bias (-0.5 g, 95%CI -2.5 to 1.5, P = 0.63). In contrast, studies published in or after 2008 were highly homogenous (I2 = 3%) and reported excellent accuracy (-0.1 g, 95%CI -2.2 to 1.9, P = 0.90).
Evaluation of the right ventricle
The complex and highly asymmetric anatomy of the right ventricle (RV) has traditionally represented a challenge for quantitative evaluation by echocardiography. Its trabeculations, along with the retrosternal position and its unique shape (defying any easy geometric approximation) explains the difficult task of RV assessment. Nonetheless, RV size and function have been shown to be important predictors of cardiovascular morbidity and mortality in patients with various conditions[14].
Conventional 2D echo lacks the possibility to encompass the whole RV structure (inflow, outflow, and apical trabecular area); consistently, RV evaluation must be pursued by means of acquisitions via multiple acoustic windows, as recommended by international guidelines[6].
Nowadays, different imaging modalities can offer better understanding of the RV anatomy (e.g., cardiac MRI, CT scan) but their use is limited by local availability, higher costs, complexity and greater time-consumption compared to echocardiography[14].
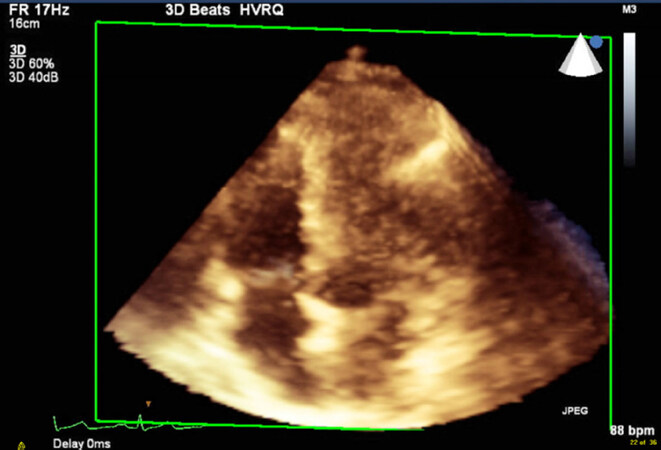
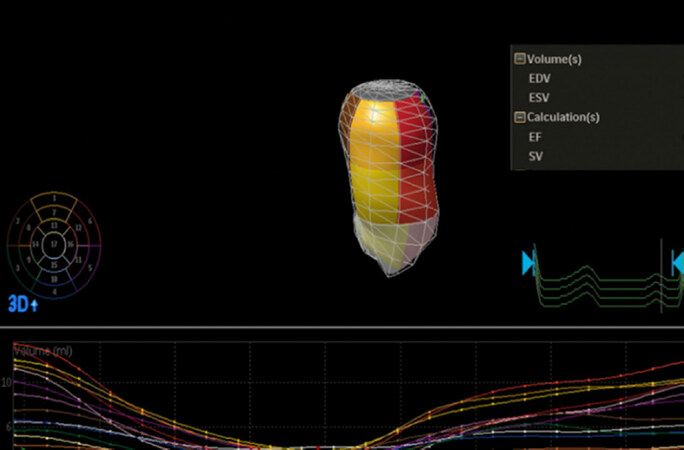
The previously described ability of 3D echo to acquire the whole structure of interest has opened to new possibilities to overcome the challenges encountered with conventional echocardiography when it comes to exploring “the forgotten ventricle” [Figure 1][15].
Vendors have developed dedicated software packages allowing the operator to perform accurate RV volumes and function evaluation with no need for geometrical assumptions.
As in the case of the LV, underestimation of RV volumes should be expected when comparing 3D echo to cardiac MRI. In a study enrolling 100 consecutive adult patients with normal or pathologic RVs, Leibundgut and associates generated a dynamic polyhedron model of the RV using a dedicated software. EDV, ESV, and stroke volumes were slightly lower on 3D echo imaging than on MRI (124.0 ± 34.4 vs. 134.2 ± 39.2 mL, P < 0.01; 65.2 ± 23.5 vs. 69.7 ± 25.5 mL, P = 0.2; and 58.8 ± 18.4 vs. 64.5 ± 24.1 mL, P < 0.1, respectively), while no significant difference was observed for EF (47.8 ± 8.5% vs. 48.2 ± 10.8%, P = 5.7)[16] confirming results of other groups[17,18].
Various imaging modalities (2D echo, 3D echo, radionucleotide ventriculography, cardiac computed tomography, gated single-photon emission CT, and invasive cardiac cine ventriculography) were tested against cardiac MRI with respect to both LVEF and RVEF accuracy by Pickett et al.[19]. For RVEF, CT and 3D echo were shown to have the best data to support their use with a bias < 5%, tight limits of agreement and good correlation coefficients (r > 0.75)[19].
Evaluation of cardiac valves
Conventional 2D echocardiography requires multiple views of the structure of interest along with the ability to mentally reconstruct the 3D image of the item under investigation, which can be particularly challenging in the case of the complex anatomy of diseased cardiac valves.
3D echo allows easier and, most importantly, better understanding of valvular anatomy and morphological disorders. These possibilities have made 3D echo extremely useful in the case of mitral valve (MV) disease, when, for instance, precise location of flail or prolapsed leaflet becomes of great importance both for diagnosis and repair.
Mitral valve
The human MV is a complex 3D device made of independent elements that constitute a dynamic structure where interaction among leaflets, mitral annulus, subvalvular apparatus (chordae tendineae and papillary muscles), and the left ventricle must be perfectly coordinated[20].
The superiority of 3D over 2D echo in the assessment of patients with mitral regurgitation (MR) has consistently been reported[21]. Pepi et al.[22] evaluated the feasibility and accuracy of 3D transthoracic echocardiography (3D TTE) and 3D TEE in evaluating MV pathology in 112 patients undergoing MV repair surgery. 3D techniques were feasible in a relatively short time (3D TTE: 7 ± 4 min; 3D TEE: 8 ± 3 min), with good (3D TTE 55%; 3D TEE 35%) and optimal (3D TTE 21%; 3D TEE 45%) imaging quality in the majority of cases. 3D TEE allowed more accurate identification (95.6% accuracy) of all MV lesions in comparison with other techniques; of note, 3D TTE and 2D TEE had similar accuracies (90% and 87%, respectively), whereas the accuracy of 2D TTE (77%) was significantly lower.
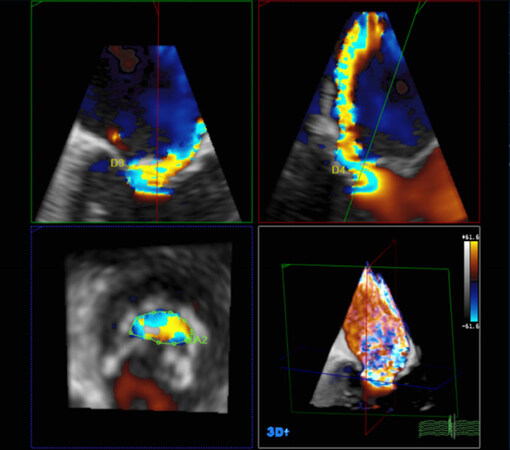
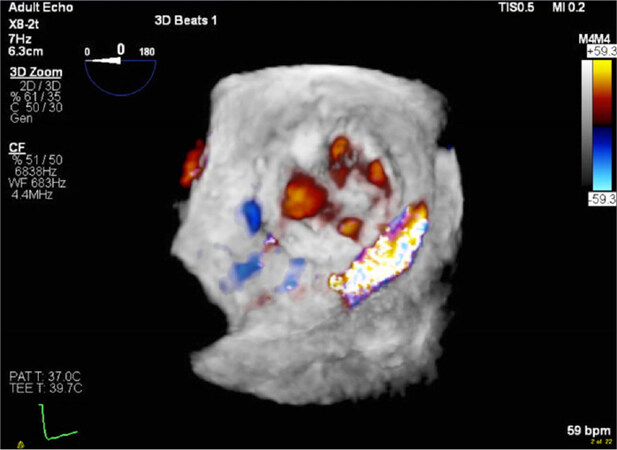
The use of 3D color enables new level of possibilities of assessment of mechanisms and severity of regurgitant lesions. Accurate evaluation of jet morphology, jet origin and jet volume is easily achieved, since direct visualization of jet characteristics is possible with no need for mental 3D reconstruction. Moreover, 3D color has prompted a true “Copernican revolution” in the quantification of the severity of MR, as it has been convincingly demonstrated that the vena contracta (VC) is often highly asymmetric [Figure 2], therefore making 2D assessment less reliable[23].
Figure 2. Transesophageal 3D-color assessment of vena contracta in a regurgitant mitral valve, showing its asymmetric shape
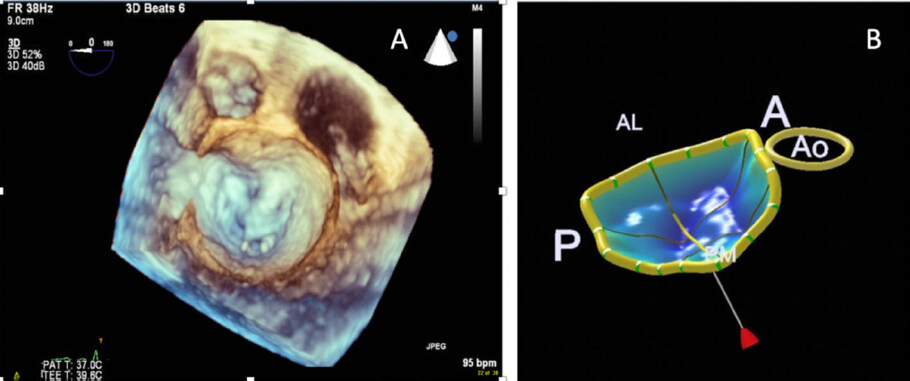
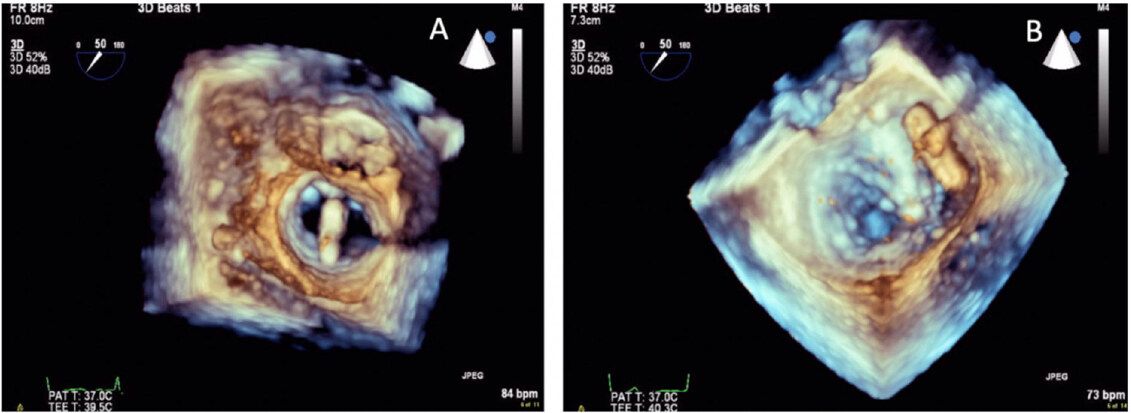
A higher level of accuracy in terms of MV anatomical details, identification of diseased segments, prolapsing or calcified scallops, measurement of leaflet surface, tethering distance, tenting volume (just to cite some of the many parameters one could collect), can be obtained by means of 3D TEE, as compared to TTE. Newly developed semi-automated/ modeling packages for quantitative analysis of MV geometry and function based on 3D echo images acquired during 3D TEE are also available [Figure 3].
Figure 3. Multi-beat transesophageal 3D view of the mitral valve in en face “surgeons view”, showing a prolapse of the A2 scallop and flail chordae (A); mitral valve navigator model of the mitral valve allowing for complex measurements of the mitral valve apparatus (B)
These tools are particularly helpful in understanding the pathophysiology and severity of MR, as well as in planning surgical or interventional treatments. In a study evaluating the utility of parametric 3D modeling of the MV, investigators found that modeling with color-encoded display of the MV leaflets, assisted the most inexperienced imagers in MV pathology diagnosis[24].
3D echo might also provide incremental diagnostic value in patients with MV stenosis. The ability of 3D echo to assess residual MV orifice area has been validated by Zamorano et al.[25]. Authors tested which echo-Doppler method showed the best agreement with MV area invasively evaluated by the Gorlin’s formula in 80 patients with rheumatic MV stenosis. Compared with all other echo-Doppler methods, real time 3D echo had the best agreement with the invasively determined MV area (average difference between both methods and limits of agreement: 0.08 cm2 [-0.48-0.6]); notably, interobserver and intraobserver variability were both good (intraclass correlation coefficient [ICC] = 0.90 and 0.96, respectively).
Aortic valve
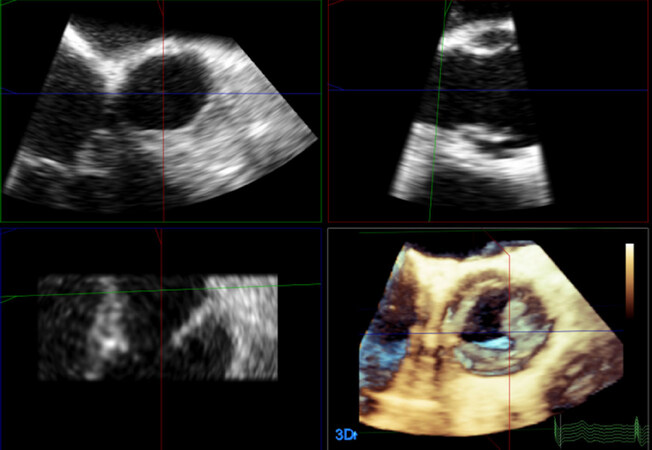
Lower rates of successful 3D echo imaging of the aortic valve as compared to the MV have been reported in the past years. In an initial experience with real-time 3D TEE on 211 patients by Sugeng et al.[21], excellent visualization of the aortic valve was achieved in only 18% of cases, as opposed to the MV for which excellent visualization was reported in 85% to 91% of patients for all scallops of both MV leaflets). Nevertheless, improved technology (both on the hardware and software sides) has nowadays made 3D echo not only capable of providing good quality images but also to overcome another pitfall intrinsic to the traditional evaluation of the aortic valve by means of 2D echo. Standard use of the continuity equation for the evaluation of aortic valve area (AVA) in patients with aortic stenosis assumes a circular shape of the LV outflow tract (LVOT)[26]. However, similarly to the VC, the LVOT can often be asymmetric [Figure 4]; this, in turn, potentially leads to significant under-estimation of the AVA when continuity equation is applied in the context of 2D imaging.
Figure 4. Transesophageal 3D echo: multi-planar reconstruction of the left ventricular outflow tract, showing its asymmetric shape
An interesting study by Gaspar and associates showed that the LVOT was eccentric in 96% of patients studied and that LVOT areas calculated from 2D echo systematically underestimated LVOT area compared to cardiac CT by 17 ± 16%[27]. However, due to inadequate TTE image quality of the LVOT, there was only moderate correlation between 3D LVOT area and cardiac CT (r = 0.63).
Multiple studies have shown good correlation between 3D annular and perimeter measurements of the aortic annulus compared to CT. In a meta-analysis by Elkaryoni and colleagues including 14 studies and 1,228 patients, there was excellent agreement between 3D TEE annular area and perimeter with CT-derived measurements (r > 0.8)[28].
There has been interest in using 3D TEE to determine AVA in aortic stenosis - both as 3D planimetry and using 3D planimetry of the LVOT and applying the continuity equation. While 2D echo assessment of AVA by planimetry and Doppler derived techniques (via the continuity equation) has been widely validated and studied, 3D AVA values have uncertain clinical significance and these values have yet to be validated.
Evaluation of aortic valve by 3D color echo may also improve the quantification of aortic regurgitation (AR) severity. Perez de Isla et al.[29] used 3D color Doppler of AR to measure 3D VC to quantify severe AR and compared it to the gold standard of MRI. They studied 32 patients and traced the cross-sectional effective orifice area by using multiplane reconstruction of the en-face view (equivalent to VC area). They found excellent linear correlation between 3D echo and MRI: At a 3D vena contracta area cut off of 0.50 cm2, the receiver operating characteristic curve demonstrated excellent area under the curve to detect severe AR (3D VC cross sectional area method = 0.97; 3D VC cross sectional area/LVOT cross sectional area method = 0.98), and the authors concluded that 3D color echo is an accurate and highly reproducible diagnostic tool for estimating AR severity. Additionally 3D color echo had better agreement with CMR than 2D color Doppler. The utility of 3D echo in the setting of AR was also explored by Pirat and colleagues, who showed that proximal isovelocity surface area by 3D color-Doppler is feasible and quantification of 3D aortic regurgitant volume was more accurate than the usual 2D methods (r = 0.83 and r = 0.69 vs. volumes measured by flow meter for 3D and 2D echo, respectively)[30].
Three-dimensional transesophageal echocardiography in the perioperative setting
As a modality, “perioperative TEE” is defined as the use of TEE for clinical care of patients before, during and immediately after procedures. 3D echo has enhanced both quantitative and qualitative information of ventricular and valvular function as well as our ability to use TEE as a procedural adjunct during structural heart cases and open cardiac surgery.
Perioperative 3D TEE assessment of left and right ventricular function
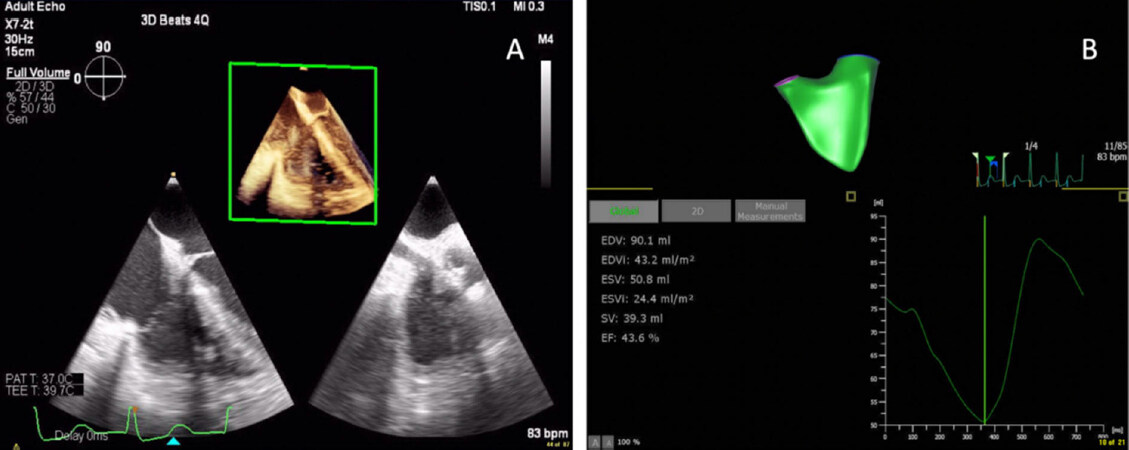
Assessment of ventricular function is one of the cornerstones of perioperative TEE. Though 2D method of disks is currently the gold standard[6], 3D has been shown to be more accurate than 2D, and 3D semi-automated assessment of LV function has recently been incorporated in the ASE guidelines for comprehensive perioperative TEE exam[31][Figure 5]. Similar to TTE, recent studies with 3D TEE have shown improved accuracy compared to 2D with the gold standard of cardiac MRI[32].
Figure 5. 3D semi-automatic quantification of the left ventricle demonstrating global function and regional wall motion
3D assessment of the RV intraoperatively is a promising new application of perioperative TEE. There is ample literature suggesting that current 2D indices of RV assessment are limited in assessing overall RV function due to the complex geometry of the right ventricle. There have been TTE studies demonstrating improved accuracy of RV quantification with 3D echo[19]. In the past, this application was limited by the fact that measurements needed to be made offline through dedicated software and were more time consuming, which is cumbersome in the acute perioperative setting. In earlier generation 3D TEE probes, the large sector size necessary to capture the entire RV created images with poor temporal resolution. However, with newer, more powerful 3D TEE probes, temporal resolution is often preserved [Figure 6] in addition to providing superior spatial resolution. Though 3D RV evaluation is feasible, there remains few intraoperative RV 3D imaging studies to date, and those that exist performed analyses off-line. Of note, a recent article by Grønlykke and colleagues comparing 2D and 3D indices of RV function found that 3D evaluation of RV stroke volume correlated with RV pulmonary artery catheter derived cardiac output[33]. Nevertheless, as 3D evaluation of the RV becomes more efficient, it is expected to be incorporated to the intraoperative workflow.
Perioperative 3D TEE for procedure guidance and valvular assessment
3D TEE has improved communication between the cardiac anesthesiologist and cardiac surgeon or interventional cardiologist by allowing the structures of interest to be shown in real-time, reconstructed in the en face “surgeon’s view” - how the surgeon sees the structure anatomically in the chest. Studies have shown that 3D imaging of the MV is superior to 2D in assessing the pathology of degenerative MR and predicting the complexity of MV repair[34-36], as well as the success of the repair[37]. As valve sparing aortic surgeries become more preferred and commonly performed, it remains to be seen whether 3D will be helpful in predicting success of aortic valve repairs[38].
Advancements in 3D technology has allowed real time 3D TEE to have a central role in percutaneous structural heart interventions. While guidance during these procedures was originally recommended for intracardiac positioning of catheters only, it now also includes assisting with the actual deployment of devices. This is crucial for percutaneous mitral and tricuspid repair, paravalvular leak closures, atrial septal defects (ASDs) closure, left atrial appendage (LAA) closure, and emerging new devices such as the transcatheter MV repair and replacement devices (TMVR). In the specific case of TMVR, 3D TEE imaging is essential for the trans-septal puncture (position ideally slightly inferior and posterior from the middle of the interatrial septum) to allow for ability to maneuver the TMVR system. If the approach is apical, TEE is used to locate the optimal site for incision. TEE is then used with fluoroscopy for positioning of the TMVR device within the native annulus or surgical ring, usually with 3D imaging or biplane imaging (which requires 3D technology) to avoid malpositioning of the TMVR during deployment[39].
3D TEE imaging’s role is pivotal with MitraClip procedures[40][Figure 7], for depth perception as well as the spatial orientation not well appreciated during fluoroscopic imaging[41].
Figure 7. Real-time 3D transesophageal echo (RT 3D TEE) to guide placement of the MitraClip device by lining the device perpendicular to the line of coaptation (A); RT 3D TEE to guide placement of the second MitraClip device (B)
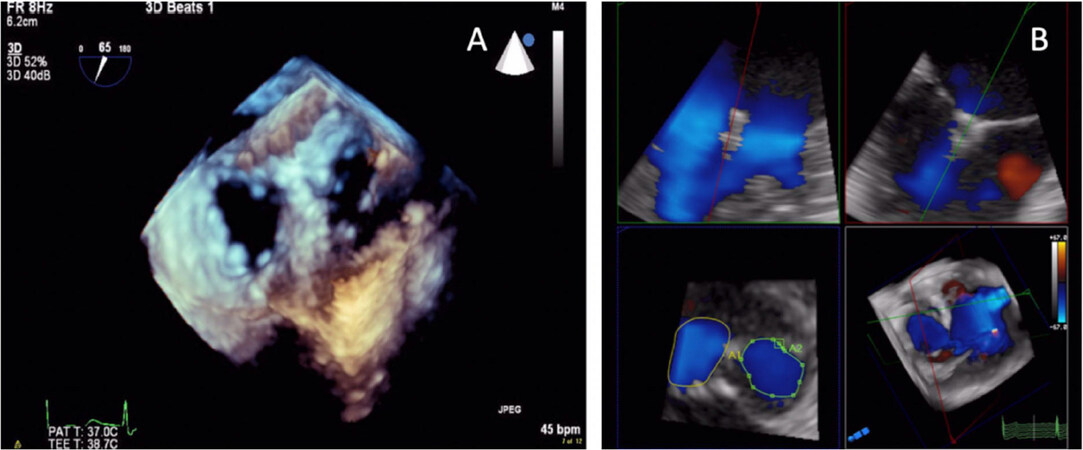
Echo and fluoroscopic images are displayed simultaneously to facilitate orientation of catheters and device positioning. Given the great utility of 3D TEE in the assessment of patients with multiple valve orifices or complex or multiple regurgitant jets, its advantages over traditional 2D TEE are self-evident when evaluating patients after MitraClip [Figure 8][42].
Figure 8. Transesophageal 3D view of the double orifice created by MitraClip, a percutaneous mitral valve repair device (A); quantitative assessment of valve area of the double orifice by MPR; Multi-planar reconstruction (B)
Assessment of severity of residual MR is central as it determines procedural success and patient prognosis after percutaneous edge-edge clip repair. Grading residual MR severity after percutaneous edge-edge clip repair is challenging and assessment has traditionally relied on integration of multiple 2D echo parameters as recommended by national and international societies[43,44].
Notably, 3D color Doppler allows for direct measurement of the effective orifice area or VC area. In a recent retrospective study enrolling 155 patients, Avenatti and colleagues evaluated the feasibility and performance of summative VC area of multiple jets for residual MR after percutaneous MV repair against expert multiparametric appraisal of MR severity and invasive hemodynamics; the authors found that summative VC area correlated well with invasive hemodynamics and that a VC area threshold of 0.27 cm2 had good diagnostic accuracy for identification of ≥ moderate MR with an area under the curve of 0.81. Additionally, smaller VC area were associated with less clinical symptoms as measured by New York Heart Association functional class improvement. This study introduces total VC area after mitral valve edge-edge clip repair as a novel technique in quantification of residual MR severity and may have potential value in Guidelines recommendations[45].
3D color Doppler has also been used to assess the location and amount of paravalvular regurgitation jets [Figure 9] both immediately and after repair, or later during paravalvular leak closure procedures[46].
Valve area immediately after repair can also be accurately assessed by 3D planimetry[42]. Multiplanar reformatting (MPR) is used for linear annular measurements of the tricuspid and mitral valves[47,48] and aids in the periprocedural sizing of rings and valves, sizing of ASDs, sizing of LAA for LAA closure devices, and valve sizing for transcatheter valve replacement[28,49,50].
3D TEE has been shown to have increased accuracy compared to 2D echo which in many cases is relevant for improved clinical outcomes. Johri et al.[51] found that in complex ASDs (33% of a total of 24 ASDs studied), ASD area measured by 3D TEE was larger than 2D TEE (2.8 ± 1.3 vs. 1.7 ± 1.4 cm2; P < 0.05). This was clinically important because 3D TEE areas were also 27% larger in patients that had residual shunt after ASD closure suggesting that 2D TEE can underestimate the area of complex-shaped ASDs. Streb and colleagues compared real time 2D TEE with real time 3D TEE in 40 patients during place of a LAA occlusion device and found that there was better device size agreement (weighted Kappa 0.62 vs. 0.28, respectively) with 3D TEE vs. 2D TEE[52]. Kronzon et al.[53] used real time 3D TEE for evaluation of residual MR after mitral repair and replacement and found that 3D TEE provided more information than 2D echo including accurate evaluation of the origin of residual MR, the type of ring or prosthesis used, and location, size, shape, and area of the dehisced segment in cases of MV replacement. This increased detail could aid in perioperative decisions to address the defect.
Future scenarios
In the future, TEE may routinely be combined with fluoroscopy for multimodality real-time fusion guidance during interventional procedures. Indeed, overlay of markers and images has the potential to improve communication between the operator and the imager, increase procedural success, and decrease radiation exposure. Future prospective studies are necessary to evaluate the utility of live fusion imaging. In addition, the advent of 3D capabilities of intracardiac echocardiographic catheters suggests that this imaging modality should be revisited for potential guidance during catheter-based interventions[54].
Until live fusion or “augmented reality” imaging is fully realized, 3D printing may be a useful option to translate CT and 3D TEE imaging. 3D printing allows for discussion and preoperative planning on patient-specific structural heart anatomy and pathology as well as preparing for 2D and 3D imaging best to elucidate the anatomy and device positioning. 3D printing may also help select device size in cases of unclear valve sizing. Currently, 3D printing is often obtained from CT or MRI modalities due to their high spatial resolution. However, 3D TEE printing is now possible, and may be applicable to future valve interventions after fine tuning work flow, 3D printing times, and software compatibility for conversion of 3D TEE to models[55].
The value of experience with 3D echo
Although 3D echo provides real-time images of the heart valves, training is required to differentiate normal anatomy from artifacts. The majority of studies demonstrating benefits in diagnosis of valve pathology have included expert readers with significant experience. Less experienced readers may not be able to accurately interpret 3D echo information. The utility and accuracy of 3D echo and MPR is dependent on user familiarity; multiple studies have shown that 3D echo measurements differ among expert vs. novice echocardiographers. Even among experts, there is a significant difference between 2D and 3D measurements suggesting that 3D measurements are not interchangeable with 2D measurements. Bouchez et al.[56] studied the difference in anterior MV leaflet length as measured by expert and novice imagers. The authors found there were systematic differences in anterior mitral leaflet length in 2D vs. 3D measurements and between beginner vs. expert imager measurements (P < 0.001 and P = 0.005, respectively). Tsang et al.[57] found both novice and intermediate readers had greater difficulty interpreting 3D images of the MV than 2D. In contrast, expert readers were very proficient at identifying mitral valve anatomy in 2D and 3D. Hien’s study looked at the diagnostic accuracy of 2D vs. 3D echo for MV prolapse and found that 3D TEE conferred an advantage to both expert and inexperienced echocardiographers[24]. Therefore there is some debate as to the advantage of 3D echo interpretation and diagnosis among different level imagers. The heterogeneity of the literature suggests there is a significant learning curve for 3D echo and variation in interpretation regarding level of expertise. It is important to note that many of the studies conferring benefit of 3D and improved expert were performed by expert readers and may not be applicable in clinical practice.
Conclusion
3D echo use, both in the clinical cardiology and perioperative settings, has increased because of its ability to add important information to the standard 2D exam and evaluate structures without geometric assumptions. Both real time 3D TEE and offline quantitative measurements from 3D acquisitions have become integral for qualitative and quantitative analysis of structures and for surgical and procedural guidance. However, many of these studies were performed under optimal conditions with expert echocardiographers. The true advantage of 3D in the everyday clinical setting may be less than reported. The next generation of echo probes with high temporal resolution, improved parametric modeling, and the emergence of new structural heart devices will continue to facilitate the use of 3D echo perioperatively and increase diagnostic abilities in the clinical setting. It is imperative that imaging echocardiographers be familiar with 3D spatial orientation of intracardiac structures as well as how to perform quantitative analysis.
Declarations
Authors’ contributionsConception, design, data acquisition, drafting, critical revising, final approval: Rong LQ, Di Franco A
Availability of data and materialsNot applicable.
Financial support and sponsorshipRong LQ is supported in part by the Foundation in Anesthesia Education and Research Mentored Clinical Research training grant.
Conflicts of interestBoth authors declared there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2019.
REFERENCES
2. Di Franco A, Ohmes LB, Gaudino M, Rong LQ, Girardi LN, et al. Serendipity and innovation: history and evolution of transthoracic echocardiography. J Thorac Dis 2017;9:S257-63.
3. Skubas NJ, Mahajan A. Intraoperative evaluation of left ventricular volume and function: it is time to usher in the third dimension. Anesth Analg 2014;118:698-700.
4. Abraham TP, Warner JG, Kon ND, Lantz PE, Fowle KM, et al. Feasibility, accuracy, and incremental value of intraoperative three-dimensional transesophageal echocardiography in valve surgery. Am J Cardiol 1997;80:1577-82.
5. Zamorano JL, Badano LP, Bruce C, Chan KL, Gonçalves A, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J 2011;32:2189-214.
6. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14.
7. Surkova E, Muraru D, Aruta P, Romeo G, Bidviene J, et al. Current clinical applications of three-dimensional echocardiography: when the technique makes the difference. Curr Cardiol Rep 2016;18:109.
8. Mor-Avi V, Sugeng L, Lang RM. Three-dimensional adult echocardiography: where the hidden dimension helps. Curr Cardiol Rep 2008;10:218-25.
9. Arai K, Hozumi T, Matsumura Y, Sugioka K, Takemoto Y, et al. Accuracy of measurement of left ventricular volume and ejection fraction by new real-time three-dimensional echocardiography in patients with wall motion abnormalities secondary to myocardial infarction. Am J Cardiol 2004;94:552-8.
10. Badano LP. The clinical benefits of adding a third dimension to assess the left ventricle with echocardiography. Scientifica (Cairo) 2014;2014:897431.
11. Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol 2012;59:1799-808.
12. Jenkins C, Leano R, Chan J, Marwick TH. Reconstructed versus real-time 3-dimensional echocardiography: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2007;20:862-8.
13. Shimada YJ, Shiota T. Meta-analysis of accuracy of left ventricular mass measurement by three-dimensional echocardiography. Am J Cardiol 2012;110:445-52.
14. Surkova E, Muraru D, Iliceto S, Badano LP. The use of multimodality cardiovascular imaging to assess right ventricular size and function. Int J Cardiol 2016;214:54-69.
15. Kossaify A. Echocardiographic assessment of the right ventricle, from the conventional approach to speckle tracking and three-dimensional imaging, and insights into the “right way” to explore the forgotten chamber. Clin Med Insights Cardiol 2015;9:65-75.
16. Leibundgut G, Rohner A, Grize L, Bernheim A, Kessel-Schaefer A, et al. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: a comparison study with magnetic resonance imaging in 100 adult patients. J Am Soc Echocardiogr 2010;23:116-26.
17. Zhang QB, Sun JP, Gao RF, Lee APW, Feng YL, et al. Feasibility of single-beat full-volume capture real-time three-dimensional echocardiography for quantification of right ventricular volume: validation by cardiac magnetic resonance imaging. Int J Cardiol 2013;168:3991-5.
18. Gopal AS, Chukwu EO, Iwuchukwu CJ, Katz AS, Toole RS, et al. Normal values of right ventricular size and function by real-time 3-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr 2007;20:445-55.
19. Pickett CA, Cheezum MK, Kassop D, Villines TC, Hulten EA. Accuracy of cardiac CT, radionucleotide and invasive ventriculography, two- and three-dimensional echocardiography, and SPECT for left and right ventricular ejection fraction compared with cardiac MRI: a meta-analysis. Eur Heart J Cardiovasc Imaging 2015;16:848-52.
20. Castillo JG, Solís J, González-Pinto A, Adams DH. Surgical echocardiography of the mitral valve. Rev Esp Cardiol 2011;64:1169-81.
21. Sugeng L, Coon P, Weinert L, Jolly N, Lammertin G, et al. Use of real-time 3-dimensional transthoracic echocardiography in the evaluation of mitral valve disease. J Am Soc Echocardiogr 2006;19:413-21.
22. Pepi M, Tamborini G, Maltagliati A, Galli CA, Sisillo E, et al. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J Am Coll Cardiol 2006;48:2524-30.
23. Buck T, Plicht B. Real-time three-dimensional echocardiographic assessment of severity of mitral regurgitation using proximal isovelocity surface area and vena contracta area method. lessons we learned and clinical implications. Curr Cardiovasc Imaging Rep 2015;8:38.
24. Hien MD, Großgasteiger M, Rauch H, Weymann A, Bekeredjian R, et al. Experts and beginners benefit from three-dimensional echocardiography: a multicenter study on the assessment of mitral valve prolapse. J Am Soc Echocardiogr 2013;26:828-34.
25. Zamorano J, Cordeiro P, Sugeng L, Perez de Isla L, Weinert L, et al. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol 2004;43:2091-6.
26. Otto C. Textbook of clinical echocardiography. 5th ed. Elsevier; 2013.
27. Gaspar T, Adawi S, Sachner R, Asmer I, Ganaeem M, et al. Three-dimensional imaging of the left ventricular outflow tract: impact on aortic valve area estimation by the continuity equation. J Am Soc Echocardiogr 2012;25:749-57.
28. Elkaryoni A, Nanda NC, Baweja P, Arisha MJ, Zamir H, et al. Three-dimensional transesophageal echocardiography is an attractive alternative to cardiac multi-detector computed tomography for aortic annular sizing: systematic review and meta-analysis. Echocardiography 2018;35:1626-34.
29. Perez de Isla L, Zamorano J, Fernandez-Golfin C, Ciocarelli S, Corros C, et al. 3D color-Doppler echocardiography and chronic aortic regurgitation: a novel approach for severity assessment. Int J Cardiol 2013;166:640-5.
30. Pirat B, Little SH, Igo SR, McCulloch M, Nosé Y, et al. Direct measurement of proximal isovelocity surface area by real-time three-dimensional color Doppler for quantitation of aortic regurgitant volume: an in vitro validation. J Am Soc Echocardiogr 2009;22:306-13.
31. Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64.
32. Meris A, Santambrogio L, Casso G, Mauri R, Engeler A, et al. Intraoperative three-dimensional versus two-dimensional echocardiography for left ventricular assessment. Anesth Analg 2014;118:711-20.
33. Grønlykke L, Korshin A, Holmgaard F, Kjøller S, Gustafsson F, et al. Severe loss of right ventricular longitudinal contraction occurs after cardiopulmonary bypass in patients with preserved right ventricular output. Int J Cardiovasc Imaging 2019; doi: 10.1007/s10554-019-01616-7.
34. Grewal J, Mankad S, Freeman WK, Click RL, Suri RM, et al. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr 2009;22:34-41.
35. Manda J, Kesanolla SK, Hsuing MC, Nanda NC, Abo-Salem E, et al. Comparison of real time two-dimensional with live/real time three-dimensional transesophageal echocardiography in the evaluation of mitral valve prolapse and chordae rupture. Echocardiogr Mt Kisco N 2008;25:1131-7.
36. Wei J, Hsiung MC, Tsai SK, Ou CH, Chang CY, et al. The routine use of live three-dimensional transesophageal echocardiography in mitral valve surgery: clinical experience. Eur J Echocardiogr 2010;11:14-8.
37. Mahmood F, Matyal R. A quantitative approach to the intraoperative echocardiographic assessment of the mitral valve for repair. Anesth Analg 2015;121:34-58.
38. Di Franco A, Rong LQ, Munjal M, Weinsaft JW, Kim J, et al. Aortic symmetry index: Initial validation of a novel preoperative predictor of recurrent aortic insufficiency after valve-sparing aortic root reconstruction. J Thorac Cardiovasc Surg 2018;156:1393-4.
39. Mackensen GB, Lee JC, Wang DD, Pearson PJ, Blanke P, et al. Role of echocardiography in transcatheter mitral valve replacement in native mitral valves and mitral rings. J Am Soc Echocardiogr 2018;31:475-90.
40. American Society of Anesthesiologists and Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology 2010;112:1084-96.
41. Nyman CB, Mackensen GB, Jelacic S, Little SH, Smith TW, et al. Transcatheter mitral valve repair using the edge-to-edge clip. J Am Soc Echocardiogr 2018;31:434-53.
42. Guarracino F, Baldassarri R, Ferro B, Giannini C, Bertini P, et al. Transesophageal echocardiography during MitraClip® procedure. Anesth Analg 2014;118:1188-96.
43. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr 2017;30:303-71.
44. Stone GW, Adams DH, Abraham WT, Kappetein AP, Généreux P, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the mitral valve academic research consortium. J Am Coll Cardiol 2015;66:308-21.
45. Avenatti E, Mackensen GB, El-Tallawi KC, Reisman M, Gruye L, et al. Diagnostic value of 3-dimensional vena contracta area for the quantification of residual mitral regurgitation after mitraclip procedure. JACC Cardiovasc Interv 2019;12:582-91.
46. Franco E, Almería C, de Agustín JA, Arreo Del Val V, Gómez de Diego JJ, et al. Three-dimensional color Doppler transesophageal echocardiography for mitral paravalvular leak quantification and evaluation of percutaneous closure success. J Am Soc Echocardiogr 2014;27:1153-63.
47. Mahmood F, Subramaniam B, Gorman JH, Levine RM, Gorman RC, et al. Three-dimensional echocardiographic assessment of changes in mitral valve geometry after valve repair. Ann Thorac Surg 2009;88:1838-44.
48. Mahmood F, Kim H, Chaudary B, Bergman R, Matyal R, et al. Tricuspid annular geometry: a three-dimensional transesophageal echocardiographic study. J Cardiothorac Vasc Anesth 2013;27:639-46.
49. Johri AM, Rojas CA, El-Sherief A, Witzke CF, Chitty DW, et al. Imaging of atrial septal defects: echocardiography and CT correlation. Heart 2011;97:1441-53.
50. Nucifora G, Faletra FF, Regoli F, Pasotti E, Pedrazzini G, et al. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: implications for catheter-based left atrial appendage closure. Circ Cardiovasc Imaging 2011;4:514-23.
51. Johri AM, Witzke C, Solis J, Palacios IF, Inglessis I, et al. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr 2011;24:431-7.
52. Streb W, Mitręga K, Podolecki T, Szymała M, Leopold-Jadczyk A, et al. Two-dimensional versus three-dimensional transesophageal echocardiography in percutaneous left atrial appendage occlusion. Cardiol J 2018; doi: 10.5603/CJ.a2018.0019.
53. Kronzon I, Sugeng L, Perk G, Hirsh D, Weinert L, et al. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol 2009;53:1543-7.
54. Silvestry FE, Kadakia MB, Willhide J, Herrmann HC. Initial experience with a novel real-time three-dimensional intracardiac ultrasound system to guide percutaneous cardiac structural interventions: a phase 1 feasibility study of volume intracardiac echocardiography in the assessment of patients with structural heart disease undergoing percutaneous transcatheter therapy. J Am Soc Echocardiogr 2014;27:978-83.
55. Mashari A, Montealegre-Gallegos M, Knio Z, Yeh L, Jeganathan J, et al. Making three-dimensional echocardiography more tangible: a workflow for three-dimensional printing with echocardiographic data. Echo Res Pract 2016;3:R57-R64.
56. Bouchez S, Mackensen GB, Mauermann E, McCleish L, Cobey F, et al. Differences in two- and three-dimensional assessment of the mitral valve by novices and experts, illustrated using anterior mitral valve leaflet length. J Cardiothorac Vasc Anesth 2019;33:1022-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Rong LQ, Di Franco A. Clinical and perioperative applications of three-dimensional echocardiography. Vessel Plus 2019;3:18. http://dx.doi.org/10.20517/2574-1209.2019.007
AMA Style
Rong LQ, Di Franco A. Clinical and perioperative applications of three-dimensional echocardiography. Vessel Plus. 2019; 3: 18. http://dx.doi.org/10.20517/2574-1209.2019.007
Chicago/Turabian Style
Rong, Lisa Q., Antonino Di Franco. 2019. "Clinical and perioperative applications of three-dimensional echocardiography" Vessel Plus. 3: 18. http://dx.doi.org/10.20517/2574-1209.2019.007
ACS Style
Rong, LQ.; Di Franco A. Clinical and perioperative applications of three-dimensional echocardiography. Vessel Plus. 2019, 3, 18. http://dx.doi.org/10.20517/2574-1209.2019.007
About This Article
Copyright
Data & Comments
Data

 Cite This Article 4 clicks
Cite This Article 4 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.