Associations of lifestyle and dietary habits with hyperlipidemia in Lebanon
Abstract
Aim: This study was designed to evaluate the effects of dietary and lifestyle habits on several blood lipid parameters in the Lebanese population.
Methods: This was a cross-sectional study for 2,000 individuals, of whom 1,003 completed the survey about their dietary and lifestyle habits. Anthropometric measurements and blood tests were performed and recorded.
Results: Up to 53.2% of the population was hypercholesterolemic. Gender and age contributed to the prevalence of high levels of low density lipoprotein cholesterol (LDL-C) or triglycerides. Prevalence of hypercholesterolemia, hypertriglyceridemia and high LDL-C levels was higher in smokers, physically inactive or those who consume fatty meat or eggs. Prevalence of hypercholesterolemia was not affected by consumption of whole milk, skimmed milk or fruits and vegetables. However, the prevalence of hypertriglyceridemia and high LDL-levels was higher in individuals who consumed whole milk, and lower in those who consumed skimmed or fruits and vegetables.
Conclusion: Hyperlipidemia affects more than half of the Lebanese population. The finding that the majority of the individuals were unaware of their lipid profile mandates warrant efforts for both patient and public education.
Keywords
Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality and morbidity in both developed and developing countries.[1] Although modifiable, hyperlipidemia remains a major risk factor for many diseases including coronary artery disease (CAD) and atherosclerosis.[2,3] Although increased levels of high density lipoprotein cholesterol (HDL-C) may play a protective role against CVD,[4,5] there is a positive correlation between increased serum levels of cholesterol, triglycerides (TG), or low density lipoprotein cholesterol (LDL-C) and CAD or atherosclerosis.[4-6] Thus, it is not surprising that the major consequence of hypercholesterolemia is atherosclerosis.[7,8] The major hallmark of atherosclerosis is the buildup of lipids, mainly cholesterol, in the vascular wall.
Atherosclerosis is the most common CVD, and conditions associated with it, such as stroke and acute myocardial infarction (AMI), continue to be the leading causes of morbidity throughout the world.[9,10] By causing a reduction in the vessel lumen, atherosclerotic plaques may dramatically diminish blood supply to tissues, which could have deleterious effects on the nourishment and oxygen supply of these tissues.[11]
Hypercholesterolemia remains a leading risk factor in both developed and developing countries.[12] Several studies have reported that a decrease in the serum levels of total cholesterol (TC) and LDL-C leads to a reduction in the risk of coronary events and is thus an effective strategy in secondary prevention.[13-17] It is also well documented that hyperlipidemia plays a major role in the development of many CVDs including atherosclerosis.[2,4,5]
There are many risk factors for atherosclerosis, such as gender, diabetes, stress, and hypertension.[18] However, hypercholesterolemia in particular is critical for the genesis of the disease as it plays a permissive role in the development of other pathogenic factors such as insulin resistance and vascular inflammation.[19] Notably, the relationship between atherosclerosis and hypercholesterolemia is affected by lifestyle factors, including physical activity and smoking.
Lebanon is considered a third-world country. Alarmingly, no large studies have been conducted to determine the prevalence of hyperlipidemia in the Lebanese population. There are no national studies showing the impact of lifestyle-related factors, such as diet, physical activity, alcohol consumption, or smoking on blood levels of lipids. Despite the findings that the Mediterranean diet may help reduce CVD risk and mortality,[20] nothing is known about this relation in Lebanon, a small Mediterranean country. Interestingly, there has been a change in the dietary habits, particularly in the 20-30 years old age group living in large cities in Lebanon.
This study was therefore undertaken to first determine the prevalence and awareness of hyperlipidemia in Lebanon. It was also designed to examine the influence of diet and lifestyle factors on lipidemia in the Lebanese population.
Methods
Study population
This cross-sectional study recruited 2,000 participants; 997 of whom were later excluded because of the existence of illness with the potential to modulate lipid profiles, such as diabetes mellitus or the use of lipid altering medications. Diabetes was defined as having a fasting glucose level of ≥ 126 mg/dL or by the use of glucose-lowering drugs. Demographically, the remaining 1,003 individuals included in the study were distributed over the different geographic regions of Lebanon. Of these subjects, 501 were females and 502 males, all reported to be healthy with unremarkable previous medical history. Surprisingly, more than 70% of the subjects were unaware of their lipids profiles. Subjects were grouped in 20-year intervals. All participants above 55-year-old were assigned to one-age interval.
Data collection and definitions
Questionnaires were distributed with instructions for self-administration and collected one week later. Incomplete questionnaires were completed during short personal interviews. Information concerning height, weight, alcohol intake, smoking, and physical activity were recorded. Measurements of height and weight were taken with the participants wearing no shoes and very light clothing. Height was measured to the nearest 0.5 cm using a standard physician’s height stadiometer. Body weight was measured to the nearest 0.1 kg using a portable balance scale. Body mass index (BMI; kg/m2) was calculated as weight (kg) divided by the square of the height (m).
Hypercholesterolemia was defined as having blood total cholesterol of ≥ 200 mg/dL.
Participants not undergoing regular physical exercise for at least 30 min twice a week were considered sedentary.
Smoking was defined as smoking an average of one or more cigarettes per day.
Nutritional data were collected and pertained to the frequency of consumption of fatty meat, entire dairy products, skimmed milk, eggs, as well as fruits and vegetables.
Informed consent was obtained for all participants, either directly (for those 18 years or older) or through the parents/legal guardians (for subjects under 18 years of age).
The protocols followed for data collection, handling, and analyzing were approved by the Research Ethics Committee at the Lebanese University.
Blood sampling and laboratory
Selected participants were asked to undergo a 12-h-fasting period, after which 5 mm of blood were collected by clean venipuncture into dry tubes (BD Vacutainer®). Plasma was separated by centrifugation (1,000 g, 4°C, 20 min). Plasma samples were frozen until analysis, which was carried out not later than 4 h after phlebotomy. Since seasonal variation in plasma cholesterol levels is well documented,[21] all blood samples were collected in the same month. Plasma lipids concentrations were measured at the Clinical Biochemistry Laboratory of Rayak Hospital, Lebanon. Total cholesterol, LDL and triglycerides levels were measured using automated enzymatic-calorimetric techniques (COBAS MIRA). LDL was calculated according to Friedewald formula as the total cholesterol minus HDL minus one-fifth triglycerides level. Final results are reported in mg/dL concentrations. The used reagents were supplied by SPINREAC.S.A.
Statistical analysis
All analyses were conducted using SPSS program by stepwise multiple regression analysis. The difference in mean values between two groups (like males versus females) was tested by Student’s t-test. A level of 5% for the P value was considered significant.
Results
Anthropometric measurements of the individuals who participated in this study are shown in Table 1. The age of the participants ranged from 15 to 87 years old, with a mean of 41.64 (± 15.9; SD) years. There was no significant difference between the mean age of males and females (41.8 vs. 41.48 years, respectively.) The mean weight of the participants was 74.15 (± 13.9; SD) kg, ranging from 36 kg to 150 kg. Their heights ranged from 142 cm to 195 cm, with a mean height of 166.46 (± 7.76; SD) cm. Both the height and weight of males were significantly higher than the females’ [Table 1]. The range of the BMI was 14.2 to 49 kg/m2, with a mean of 26.703 ± 4.42 kg/m2. Age-adjusted BMI was found to be significantly higher in females than males (P < 0.05).
Characteristics of the study participants
| Males (502) | Females (501) | Total (1,003) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | ||
| Age | 41.8 | 15.9 | 71 | 41.5 | 15.8 | 71 | 41.6 | 15.9 | 72 | 0.34 |
| Height | 169.1 | 7.5 | 53 | 163.8 | 7.1 | 48 | 166.6 | 7.8 | 53 | < 0.001 |
| Weight | 77.6 | 13.1 | 100.8 | 70.9 | 13.8 | 110 | 74.2 | 13.9 | 53 | < 0.001 |
| BMI | 27.5 | 4.1 | 34.9 | 26.3 | 4.7 | 32.7 | 26.7 | 4.4 | 34.9 | # |
In this population, the means for serum levels of cholesterol, LDL-C, HDL-C and triglycerides were respectively 208.5 ± 49.5, 133.2 ± 46.1, 43.1 ± 8.9, and 163.6 ± 111.6 mg/dL [Table 2]. Overall, an alarming 53.24% of the population had a cholesterol levels above 200 mg/dL, and thus can be considered hypercholesterolemic.[22]
Means of lipid profile parameters according to sex distribution
| Males (502) | Females (501) | Total (1,003) | P-value | |
|---|---|---|---|---|
| Cholesterol | 205.7 ± 47.8 | 211.2 ± 51.9 | 208.5 ± 49.9 | 0.08 |
| LDL-C | 123.2 ± 46.3 | 140.7 ± 44.6 | 133.2 ± 46.1 | < 0.001 |
| HDL-C | 43.4 ± 8.7 | 42.9 ± 9.1 | 43.1 ± 8.9 | 0.465 |
| TG | 177.6 ± 123.4 | 150.8 ± 98.7 | 163.6 ± 111.6 | < 0.001 |
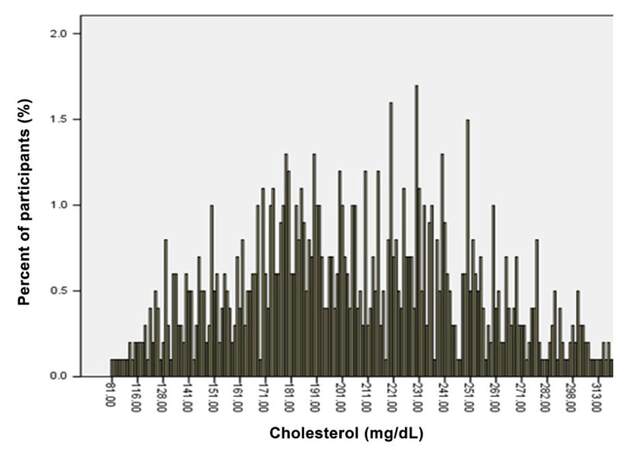
Importantly, cholesterol levels of the study participants were found to conform to a normal distribution with a mean of 208.5 ± 49.9 mg/dL, and a range of 110.4 mg/dL to 306.2 mg/dL [Figure 1]. Conformity of the distribution was tested by classical statistical analysis of the variance (q2) with confidence interval of 95% (q2c = 0.3693, q2α = 5.991, α = 0.05).
Figure 1. Distribution of cholesterol level in the population studied. The percent of the population with a measured level of cholesterol (mg/dL) is plotted
Table 2 also shows that the mean levels of cholesterol and HDL-C did not significantly differ between both genders. It is interesting to note that whereas mean values of LDL-C were significantly higher in females than males (140 vs. 123; P < 0.001), TG exhibited an opposite profile, being higher in males than females (177.6 vs. 150.8; P < 0.001).
Because gender did not significantly contribute to the levels of total cholesterol or HDL-C, we then focused our analysis on TG and LDL-C levels and sought to determine if age contributes to the observed gender differences.
Interestingly, LDL-C as well as TG levels showed a statistically significant increase with age in both males and females (P < 0.05 for TG and P < 0.001 for LDL-C). TG levels were higher in males in the two age groups [15-35] and [35-55], whereas LDL-C were higher in females in these two age groups. Interestingly, in the ≥ 55 age group, there was no significant difference in either of LDL-C or TG levels [Table 3].
Means of serum levels of cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides in males and females according to different age groups
| Age interval (years) | Gender/number | Triglycerides level | LDL level |
|---|---|---|---|
| [15-35] | Male/189 | 132.9 ± 117.2 | 109.7 ± 37.3 |
| Female/197 | 96.4 ± 48.6 | 123.9 ± 36.4 | |
| P value | < 0.001 | 0.0001 | |
| [35-55] | Male/211 | 183.1 ± 114.6 | 118.4 ± 39.5 |
| Female/190 | 159.0 ± 89.0 | 147.6 ± 45.5 | |
| P value | < 0.001 | < 0.001 | |
| ≥ 55 | Male/102 | 228.5 ± 125.9 | 142.9 ± 54.8 |
| Female/114 | 210.3 ± 122.1 | 151.2 ± 46.4 | |
| P value | 0.283 | 0.23 |
The prevalence of high levels of cholesterol, TG or LDL-C in the different dietary or lifestyle-related groups is shown in Table 4.
Prevalence of hypercholesterolemia, high triglyceridemia and high LDL-C among individuals with different lifestyle and dietary habits
| Percent of population (%) | Prevalence of hypercholesterolemia (%) | Prevalence of high triglyceride (%) | Prevalence of high LDL (%) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 50.05 | 51.3 | 56.6 | 36 |
| Female | 49.99 | 48.7 | 43.3 | 64 |
| P < 0.01 | P < 0.01 | P < 0.01 | ||
| Cigarette | ||||
| Non-smokers | 62.4 | 45.12 | 40.6 | 36.6 |
| Smokers | 37.6 | 70.02 | 50.4 | 63.4 |
| P < 0.01 | P = 0.002 | P < 0.01 | ||
| Physical activity | ||||
| Sedentary | 68.4 | 64.4 | 89.4 | 79.1 |
| Active | 31.6 | 32.9 | 10.6 | 20.9 |
| P < 0.01 | P < 0.001 | P < 0.001 | ||
| Fatty meat | ||||
| ≥ 3 servings/day | 50.1 | 72.1 | 60.2 | 53.3 |
| < 3 servings/day | 49.3 | 37.4 | 39.8 | 46.5 |
| P < 0.01 | P < 0.001 | P = 0.01 | ||
| Whole milk | ||||
| ≥ 1 servings/day | 91.9 | 55.8 | 98.2 | 84.7 |
| < 1 serving/day (on average) | 8.1 | 46.9 | 1.8 | 15.3 |
| P = 0.122 | P < 0.001 | P < 0.001 | ||
| Skimmed milk | ||||
| ≥ 1 serving/day | 10 | 55.3 | 4.5 | 15.3 |
| < 1 serving/day | 90 | 45.9 | 95.5 | 84.7 |
| P = 0.087 | P < 0.001 | P < 0.001 | ||
| Eggs | ||||
| ≥ 4-7 servings/week | 74 | 58.1 | 80.5 | 77.9 |
| < 3 servings/week | 26 | 43.9 | 19.5 | 22.1 |
| P < 0.001 | P < 0.001 | P < 0.001 | ||
| Fruits and vegetables | ||||
| ≥ 3 servings/day | 98 | 45.5 | 1.8 | 3.5 |
| < 3 servings/day | 2 | 56.4 | 98.2 | 96.5 |
| P = 0.33 | P < 0.001 | P < 0.001 | ||
The prevalence of hypercholesterolemia and triglyceridemia was significantly higher in males than females (both P < 0. 01), but prevalence of high LDL-C was higher in females (P < 0.01).
Cigarette smokers have significantly higher prevalence of high cholesterol (P < 0.01), TG (P = 0.002) and LDL-C levels (P < 0.001). Likewise, sedentary individuals exhibited higher prevalence of all the three hyperlipidemias (P < 0.01 for cholesterol and P < 0.001 for TG and LDL-C).
With respect to the dietary factors, consumption of 3 or more servings of either fatty meat or eggs was significantly associated with higher prevalence of the three hyperlipidemias. Consumption of whole or skimmed milk did not significantly affect the prevalence of hypercholesterolemia. However, participants who reportedly consumed 1 or more servings of skimmed milk had significantly lower incidence of high TG or LDL-C levels (P < 0.001). The exact opposite is noted for those who consumed 1 or more servings of whole milk; these individuals had significantly higher prevalence of high TG and LDL-C levels (P < 0.001).
The number of serving of fruits/vegetables per week did not seem to contribute to the prevalence of hypercholesterolemia. However, those who consumed 3 or more servings of fruits/vegetables had a significantly lower prevalence of high TG and LDL-C levels (P < 0.001).
Discussion
To the best of our knowledge, this is the first study to determine the prevalence of hypercholesterolemia in Lebanon. In fact, it is also the largest and most comprehensive study examining a health-related issue in Lebanon, a small country with a total population of around 4 million people. The relatively large number of participants and their wide demographic distribution covering all parts of Lebanon are two strengths of this study.
In this study, we first determined the prevalence of TC, LDL-C, HDL-C and TG in the Lebanese population. We then evaluated how age, gender, some dietary and lifestyle-related habits modulated the lipid profile. We herein report that 53% of the Lebanese population is hypercholesterolemic. Alarmingly, most of these subjects were unaware of their lipid profile. This mandates concerted efforts to educate the public about the danger of dyslipidemia as well as its relation to various cardiovascular diseases. Unfortunately, no large-scale studies have determined the CVD-associated mortality and morbidity in Lebanon. Moreover, given the lack of sufficient available literature, we could not find a study that analyzed the effect of gender or dietary/lifestyle habits on the prevalence of atherosclerosis in the Lebanese population. This has hindered us from making a meaningful conclusion on the interplay between these parameters and atherosclerosis.
Compared with other countries, the prevalence of hypercholesterolemia in Lebanon is higher than that in Turkey,[23] Saudi Arabia,[24] India,[25] Guadeloupe,[26] but lower than that in England,[27] and similar to that in Finland,[28] and the USA.[29]
Gender appeared to play an important role in the prevalence of the lipidemia studied. Although there was no difference in mean cholesterol levels between males and females, more males suffered from hypercholesterolemia. In addition, prevalence of high TG was higher in males than females. This lower prevalence in females may suggest that female sex hormones, particularly estrogen, offers a protective effect against the elevation of cholesterol or TG levels in the Lebanese population. Indeed, such a protective role for estrogen has been previously reported, but remains controversial.[30-33] Interestingly, in the female population included in this study, the levels of cholesterol and TG increased with age, even beyond menopause. On the other hand, it is possible that estrogen contributes to the increased levels of TG. While this would be consistent with other reports where estrogen was shown to precipitate hypertriglyceridemia,[34] it remains inconsistent with other studies suggesting that menopausal women have higher levels of LDL-C and TC compared with pre-menopausal women.[35,36] Moreover, estrogen injection has been shown to reduce hepatic cholesterol synthesis thereby leading to decreased blood levels of LDL-C.[36,37]
It is also possible that the male hormone, testosterone, predisposes men for hypercholesterolemia and triglyceridemia. Indeed, some reports suggest that testosterone levels are inversely proportional to serum lipid levels in males.[38] Moreover, animal studies show that testosterone-deficient male mice exhibit higher cholesterol levels, clearly suggesting a favorable role for testosterone in regulating blood lipid levels.[39] However, testosterone is unlikely to explain the higher prevalence of hypercholesterolemia in males, particularly because the population average of cholesterol did not differ between males and females.
Because it increased the prevalence of hypercholesterolemia and triglyceridemia, smoking may explain the higher prevalence of these dyslipidemias in males versus females. This would be supported by our observation that smoking is more common among men than women in the study participants. This is not surprising since smoking is still not socially very acceptable for women in some regions of Lebanon, particularly in some rural areas. Surprisingly, when we adjusted for smoking, females were found to be at higher risk of developing hyperlipidemias. However, it is important to note that the prevalence of hyperlipidemia is not always higher in smokers versus non-smokers. For instance, the prevalence of hyperlipidemia among smokers was not significantly higher than that in non-smokers in an Asian population.[40] On the contrary, in a Romanian population, current smokers appear to have a worse lipid profile in both men and women.[41]
Contrary to cholesterol’s and TG’s prevalence, LDL-C was more prevalent in females than males. This may be due to the higher BMI in females [Table 1]. Indeed, we observed that high levels LDL-C are more prevalent in individuals with higher BMI (data not shown). This is consistent with the well-known notion that high BMI correlates with unfavorable lipid profile.[42,43]
Levels of LDL-C and TG increased with age, in both males and females. However, the sex difference in the values of LDL-C and TG becomes insignificant in the oldest age group (≥ 55 years). This could be explained by the aforementioned presumed effects of estrogen and/or testosterone, since both of these hormones dramatically decrease with age. Importantly and perhaps not unexpectedly, the vast majority (> 89%) of individuals within this age group were sedentary. We report herein that prevalence of high TG or LDL-C levels is lower in individuals with a physically active lifestyle. Taken together, these findings suggest that the sedentary lifestyle in this age group may account, at least in part, for the insignificant differences between both genders.
The levels of HDL-C remained relatively constant in all age groups, regardless of the gender (data not shown). This is consistent with what is previously reported in other population groups.[23,44]
Data on the impact of egg consumption on lipid profile is inconsistent. Some report a positive association between egg consumption and unfavorable lipid profile; while others suggest a negative or no association.[45-49] Because of this inconsistency, we investigated whether egg consumption affects the prevalence of different hyperlipidemias. The prevalence of hypercholesterolemia, hypertriglyceridemia and elevated LDL-C was higher in the group where egg consumption was ≥ 4 servings/week. Therefore, in the Lebanese population, consuming an average of one or more eggs per day is likely to negatively impact lipid profile. It is important to note that egg consumption itself is reported to increase intake of total fat or cholesterol.[50] Further studies are warranted to determine if this effect is modulated by other factors, like gender, age, or other dietary and lifestyle related factors.
Previous studies have suggested that consumption of skimmed milk does not modulate serum cholesterol level.[51] However, another study showed lower levels of serum cholesterol and LDL-C after isocaloric substitution of whole with skimmed milk.[52] In this report, we found that consumption of whole milk or skimmed milk did not affect the prevalence of hypercholesterolemia. Nonetheless, consumption of skimmed milk decreased the prevalence of high TG and high LDL-C but whole milk consumption increased this prevalence.
Consumption of a diet rich in fruits and vegetables is reported to favorably modulate the lipid profile in humans.[53] Indeed, high fruit intake is significantly associated with reduced odds of hypertriglyceridemia.[54] This is further supported by a recent study in the Korean population showing strong association between fruit intake and reduced prevalence of hypertriglyceridemia.[55] Interestingly, this study reported no association between the consumption of vegetables and blood lipid levels.[55] However, several other studies report little or no effects of fruits and vegetable consumption on lipid profile.[56-58] More recently, soya products were shown to favorably modulate lipid levels of TC, TG, and LDL-C and HDL-C.[59] These seemingly paradoxical results could be the result of different study designs as well as the data analysis.[60] It may also depend on whether the consumed vegetables are cooked/boiled or not, as boiling reduces some of the bioactive phytochemicals.[61] In this study, we found that the prevalence of hypertriglyceridemia and elevated LDL-C was lower in the group with the high consumption of fruits and vegetables.
It was strikingly surprising that most of the participants were unaware of their lipid profiles. This is perhaps owing to the absence of effective patient and public education. This is also in line with other reports indicating that some types of dyslipidemias are both underdiagnosed and under-treated.[62] It is hoped that this study will increase the awareness of the Lebanese community with respect to hyperlipidemia and its detrimental consequences. It is recommended that patient education as well as national programs be put in place to further reduce the major risk factors of CVD.
Declarations
Authors’ contributionsContributed to the collection of data: A.A. Samaha, M. Gebbawi, M. Fawaz, R. Houjayri, R. Samaha, S. Baydoun
Analyzed the results: A.H. Eid, F. Zouein
Wrote the manuscript: A.H. Eid
AcknowledgmentsThe authors would like to thank all those who helped in dealing with the patients, specimen collection, etc. for their assistance.
Financial support and sponsorshipNone.
Conflicts of interestThere are no conflicts of interest.
Patient consentInformed consent was obtained for all participants, either directly (for those 18 years or older) or through the parents/legal guardians (for subjects under 18 years of age).
Ethics approvalThe protocols followed for data collection, handling, and analyzing were approved by the Research Ethics Committee at the Lebanese University and Makassed General Hospital.
REFERENCES
1. World Health Organization. Cardiovascular diseases (CVDs). In: Fact Sheets: World Health Organization; 2017.
2. Wilson P, D'Agostino R, Levy D, Belanger A, Silbershatz H, Kannel W. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47.
3. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen MR, Tokgözoglu L, Tybjærg-Hansen A, Watts GF; European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345-61.
4. National Cholesterol Education Program (NCEP) Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421.
5. Gordon D, Probstfield J, Garrison R, Neaton J, Castelli W, Knoke J, Jacobs DJ, Bangdiwala S, Tyroler H. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8-15.
6. Musunuru K, Kathiresan S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ Res 2016;118:579-85.
7. Daugherty A, Tabas I, Rader DJ. Accelerating the pace of atherosclerosis research. Arterioscler Thromb Vasc Biol 2015;35:11-2.
8. Melendez QM, Krishnaji ST, Wooten CJ, Lopez D. Hypercholesterolemia: the role of PCSK9. Arch Biochem Biophys 2017;625-626:39-53.
9. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28-e292.
10. Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res 2016;118:535-46.
11. Saleh Al-Shehabi T, Iratni R, Eid AH. Anti-atherosclerotic plants which modulate the phenotype of vascular smooth muscle cells. Phytomedicine 2016;23:1068-81.
12. World Health Organization. World Health Report 2002. Available from: http://www.who.int/whr/2002/en/. [Last accessed on Jul 31, 2017].
13. Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, Williams PT, Johnstone IM, Champagne MA, Krauss RM. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation 1994;89:975-90.
14. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ; Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004;44:720-32.
15. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM, Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615-22.
16. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996;335:1001-9.
17. Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, Pyörälä K, Miettinen T, Wilhelmsen L, Olsson AG, Wedel H; Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl 2004;5:81-7.
18. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262-75.
20. Keys A, Aravanis C. Seven countries : a multivariate analysis of death and coronary heart disease. Cambridge: Harvard Univ. Press; 1980.
21. Råstam L, Hannan PJ, Luepker RV, Mittelmark MB, Murray DM, Slater JS. Seasonal variation in plasma cholesterol distributions: implications for screening and referral. Am J Prev Med 1992;8:360-6.
22. Gupta R, Rao RS, Misra A, Sharma SK. Recent trends in epidemiology of dyslipidemias in India. Indian Heart J 2017;69:382-92.
23. Erem C, Hacihasanoglu A, Deger O, Kocak M, Topbas M. Prevalence of dyslipidemia and associated risk factors among Turkish adults: Trabzon lipid study. Endocrine 2008;34:36-51.
24. al-Nuaim A, al-Rubeaan K, al-Mazrou Y, al-Attas O, al-Daghari N. Prevalence of hypercholesterolemia in Saudi Arabia, epidemiological study. Int J Cardiol 1996;54:41-9.
25. Singh R, Sharma J, Rastogi V, Raghuvanshi R, Moshiri M, Verma S, Janus E. Prevalence of coronary artery disease and coronary risk factors in rural and urban populations of north India. Eur Heart J 1997;18:1728-35.
26. Foucan L, Kangambega P, Koumavi Ekouévi D, Rozet J, Bangou-Brédent J. Lipid profile in an adult population in Guadeloupe. Diabetes Metab 2000;26:473-80.
27. Primatesta P, Poulter N. Lipid concentrations and the use of lipid lowering drugs: evidence from a national cross sectional survey. BMJ 2000;321:1322-5.
28. Vartiainen E, Jousilahti P, Alfthan G, Sundvall J, Pietinen P, Puska P. Cardiovascular risk factor changes in Finland, 1972-1997. Int J Epidemiol 2000;29:49-56.
29. Ford E, Li C, Pearson W, Zhao G, Mokdad A. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol 2010;140:226-35.
30. Kavanagh K, Davis M, Zhang L, Wilson M, Register T, Adams M, Rudel L, Wagner J. Estrogen decreases atherosclerosis in part by reducing hepatic acyl-CoA:cholesterol acyltransferase 2 (ACAT2) in monkeys. Arterioscler Thromb Vasc Biol 2009;29:1471-7.
31. Godsland I. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: analysis of studies published from 1974-2000. Fertil Steril 2001;75:898-915.
32. Li H, Mani S, Wu L, Fu M, Shuang T, Xu C, Wang R. The interaction of estrogen and CSE/H2S pathway in the development of atherosclerosis. Am J Physiol Heart Circ Physiol 2017;312:H406-14.
33. Oh Y, Jin Y, Park Y. Synergic hypocholesterolaemic effect of n-3 PUFA and oestrogen by modulation of hepatic cholesterol metabolism in female rats. Br J Nutr 2015;114:1766-73.
34. Lee J, Goldberg I. Hypertriglyceridemia-induced pancreatitis created by oral estrogen and in vitro fertilization ovulation induction. J Clin Lipidol 2008;2:63-6.
35. Phan BA, Toth PP. Dyslipidemia in women: etiology and management. Int J Womens Health 2014;6:185-94.
36. Moorthy K, Yadav UC, Mantha AK, Cowsik SM, Sharma D, Basir SF, Baquer NZ. Estradiol and progesterone treatments change the lipid profile in naturally menopausal rats from different age groups. Biogerontology 2004;5:411-9.
37. Nigro M, Santos AT, Barthem CS, Louzada RA, Fortunato RS, Ketzer LA, Carvalho DP, de Meis L. A change in liver metabolism but not in brown adipose tissue thermogenesis is an early event in ovariectomy-induced obesity in rats. Endocrinology 2014;155:2881-91.
38. Zhang N, Zhang H, Zhang X, Zhang B, Wang F, Wang C, Zhao M, Yu C, Gao L, Zhao J, Guan Q. The relationship between endogenous testosterone and lipid profile in middle-aged and elderly Chinese men. Eur J Endocrinol 2014;170:487-94.
39. Hatch NW, Srodulski SJ, Chan HW, Zhang X, Tannock LR, King VL. Endogenous androgen deficiency enhances diet-induced hypercholesterolemia and atherosclerosis in low-density lipoprotein receptor-deficient mice. Gend Med 2012;9:319-28.
40. Kim DE, Lee KB, Jang IM, Roh H, Ahn MY, Lee J. Associations of cigarette smoking with intracranial atherosclerosis in the patients with acute ischemic stroke. Clin Neurol Neurosurg 2012;114:1243-7.
41. Popa SG, Mota M, Mihaltan FD, Popa A, Munteanu I, Mota E, Serafinceanu C, Guja C, Hancu N, Catrinoiu D, Lichiardopol R, Bala C, Mihai B, Radulian G, Roman G, Timar R. Associations of smoking with cardiometabolic profile and renal function in a Romanian population-based sample from the PREDATORR cross-sectional study. Eur J Gen Pract 2017;23:164-70.
42. Donahue R, Orchard T, Kuller L, Drash A. Lipids and lipoproteins in a young adult population. The Beaver County Lipid Study. Am J Epidemiol 1985;122:458-67.
43. Yang Z, Ding X, Liu J, Duan P, Si L, Wan BH, Tu P. Associations between anthropometric parameters and lipid profiles in Chinese individuals with age ≥ 40 years and BMI < 28kg/m2. Plos One 2017;12:e0178343.
44. Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet 1992;339:1128-30.
45. Herron K, McGrane M, Waters D, Lofgren I, Clark R, Ordovas J, Fernandez M. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J Nutr 2006;136:1161-5.
46. Chakrabarty G, Manjunatha S, Bijlani R, Ray R, Mahapatra S, Mehta N, Lakshmy R, Vashisht S, Manchanda S. The effect of ingestion of egg on the serum lipid profile of healthy young Indians. Indian J Physiol Pharmacol 2004;48:286-92.
47. Fernandez M. Dietary cholesterol provided by eggs and plasma lipoproteins in healthy populations. Curr Opin Clin Nutr Metab Care 2006;9:8-12.
48. Chakrabarty G, Bijlani R, Mahapatra S, Mehta N, Lakshmy R, Vashisht S, Manchanda S. The effect of ingestion of egg on serum lipid profile in healthy young free-living subjects. Indian J Physiol Pharmacol 2002;46:492-8.
49. Ballesteros M, Cabrera R, Saucedo MS, Fernandez M. Dietary cholesterol does not increase biomarkers for chronic disease in a pediatric population from northern Mexico. Am J Clin Nutr 2004;80:855-61.
50. Choi Y, Chang Y, Lee JE, Chun S, Cho J, Sung E, Suh BS, Rampal S, Zhao D, Zhang Y, Pastor-Barriuso R, Lima JA, Shin H, Ryu S, Guallar E. Egg consumption and coronary artery calcification in asymptomatic men and women. Atherosclerosis 2015;241:305-12.
51. Hussi E, Miettinen T, Ollus A, Kostiainen E, Ehnholm C, Haglund B, Huttunen J, Manninen V. Lack of serum cholesterol-lowering effect of skimmed milk and butter milk under controlled conditions. Atherosclerosis 1981;39:267-72.
52. Estévez-González M, Saavedra-Santana P, Betancor-León P. Reduction of serum cholesterol and low-density lipoprotein cholesterol levels in a juvenile population after isocaloric substitution of whole milk with a milk preparation (skimmed milk enriched with oleic acid). J Pediatr 1998;132:85-9.
53. Smith-Warner SA, Elmer PJ, Tharp TM, Fosdick L, Randall B, Gross M, Wood J, Potter JD. Increasing vegetable and fruit intake: randomized intervention and monitoring in an at-risk population. Cancer Epidemiol Biomarkers Prev 2000;9:307-17.
54. Hong S, Song Y, Lee KH, Lee HS, Lee M, Jee SH, Joung H. A fruit and dairy dietary pattern is associated with a reduced risk of metabolic syndrome. Metabolism 2012;61:883-90.
55. Yuan C, Lee HJ, Shin HJ, Stampfer MJ, Cho E. Fruit and vegetable consumption and hypertriglyceridemia: Korean National Health and Nutrition Examination Surveys (KNHANES) 2007-2009. Eur J Clin Nutr 2015;69:1193-9.
56. Broekmans W, Klöpping-Ketelaars W, Kluft C, van den Berg H, Kok F, van Poppel G. Fruit and vegetables and cardiovascular risk profile: a diet controlled intervention study. Eur J Clin Nutr 2001;55:636-42.
57. Zino S, Skeaff M, Williams S, Mann J. Randomised controlled trial of effect of fruit and vegetable consumption on plasma concentrations of lipids and antioxidants. BMJ 1997;314:1787-91.
58. Djuric Z, Ren J, Mekhovich O, Venkatranamoorthy R, Heilbrun L. Effects of high fruit-vegetable and/or low-fat intervention on plasma micronutrient levels. J Am Coll Nutr 2006;25:178-87.
59. Tokede OA, Onabanjo TA, Yansane A, Gaziano JM, Djousse L. Soya products and serum lipids: a meta-analysis of randomised controlled trials. Br J Nutr 2015;114:831-43.
60. Dauchet L, Amouyel P, Dallongeville J, Medscape. Fruits, vegetables and coronary heart disease. Nat Rev Cardiol 2009;6:599-608.
61. Moreno DA, Lopez-Berenguer C, Garcia-Viguera C. Effects of stir-fry cooking with different edible oils on the phytochemical composition of broccoli. J Food Sci 2007;72:S064-8.
62. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Borén J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjærg-Hansen A; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Eur Heart J 2013;34:3478-90a.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Samaha AA, Zouein F, Gebbawi M, Fawaz M, Houjayri R, Samaha R, Baydoun S, Eid AH. Associations of lifestyle and dietary habits with hyperlipidemia in Lebanon. Vessel Plus 2017;1:98-106. http://dx.doi.org/10.20517/2574-1209.2017.18
AMA Style
Samaha AA, Zouein F, Gebbawi M, Fawaz M, Houjayri R, Samaha R, Baydoun S, Eid AH. Associations of lifestyle and dietary habits with hyperlipidemia in Lebanon. Vessel Plus. 2017; 1: 98-106. http://dx.doi.org/10.20517/2574-1209.2017.18
Chicago/Turabian Style
Samaha, Ali A., Fouad Zouein, Maya Gebbawi, Mirna Fawaz, Raheel Houjayri, Rana Samaha, Safaa Baydoun, Ali H. Eid. 2017. "Associations of lifestyle and dietary habits with hyperlipidemia in Lebanon" Vessel Plus. 1: 98-106. http://dx.doi.org/10.20517/2574-1209.2017.18
ACS Style
Samaha, AA.; Zouein F.; Gebbawi M.; Fawaz M.; Houjayri R.; Samaha R.; Baydoun S.; Eid AH. Associations of lifestyle and dietary habits with hyperlipidemia in Lebanon. Vessel Plus. 2017, 1, 98-106. http://dx.doi.org/10.20517/2574-1209.2017.18
About This Article
Copyright
Data & Comments
Data
 Cite This Article 14 clicks
Cite This Article 14 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.