Adipose tissue-derived cytokines, CTRPs as biomarkers and therapeutic targets in metabolism and the cardiovascular system
Abstract
Increasing evidence indicates that adipose tissue-originated cytokines mediate communication between obesity-related exogenous molecules and the molecular events that initiate the metabolic syndrome and the inflammatory responses in the cardiovascular system (CVS). Adipose tissue-derived cytokines including the C1q/tumor necrosis factor-related proteins (CTRPs), a 15-member family of novel adipokines, has attracted much interest due to its metabolic regulatory and anti-inflammatory effect and appear to play a pathophysiological role in metabolism and immunity. To date, 15 members of CTRP family have been identified and they also play a role in the CVS, especially as potent biomarkers or therapeutic targets for modulating metabolic or inflammatory functions. Therefore, this review will focus on the characteristics of CTRPs that influence cardiovascular function and on the potential of CTRPs as biomarkers and therapeutic targets.
Keywords
Introduction
During the last decade, a mounting number of adipocyte-originated hormones (adipokines) have been recognized and documented to be discriminately regulated during the onset of metabolic syndrome, obesity, and the inflammatory processes. By acting systemically as circulating hormones or locally on diverse cell types, adipokines are involved in the regulation of many physiological functions, including energy storage, metabolism, and the development of obesity-associated disorders (type 2 diabetes mellitus, cardiovascular disease, the inflammatory process).
Adiponectin, discovered as the first member of the C1q/tumor necrosis factor-related proteins (CTRPs) family, has been broadly investigated because of its metabolic regulatory and cardiovascular protective effects. To date, additional CTRP family members, a newly discovered novel family of adipokines, have been identified that may play a role in metabolism and cardiovascular system path-physiological regulation. Hence, in future, the studies on these novel adipokines will provide new understandings into the physiological/pathological mechanisms including the communication of different organs based on energy homeostasis, homeostasis of the internal environment, and prevention of inflammation.
The current review focuses on the main roles of CTRPs in the context of pathophysiology with particular attention to their potential biomarker features and therapeutic roles in the metabolic energy balance regulation, inflammatory responses and in obesity-associated disorders.
What are CTRPs?
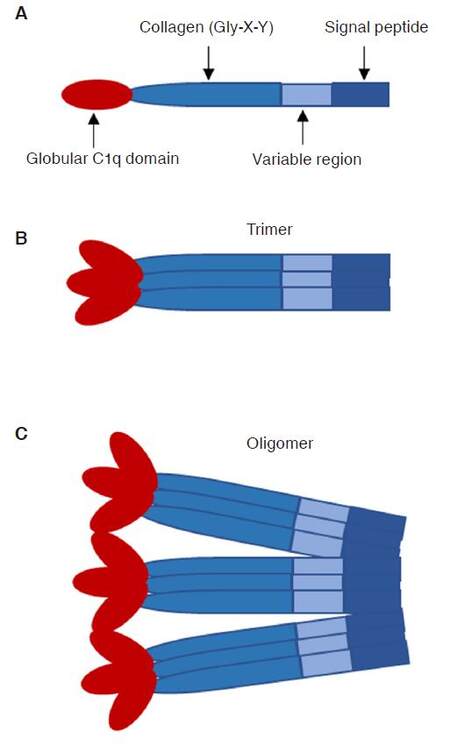
CTRPs, first identified by the Harvey Lodish laboratory, are considered a new family of secreted proteins from various organs. These 15 secreted proteins (CTRP1 to CTRP15) share common structural and functional characteristics with adiponectin: a N-terminal signal peptide, a short variable region, a collagen domain containing collagen triples (Gly-X-Y), and a C-terminal globular complement factor C1q domain [Figure 1][1,2]. CTRPs display distinct biological and signaling properties by occurring in the circulation as trimers and assembling with their basic structural unit into hexameric and high molecular weight oligomeric complexes [Figure 1]. Most CTRPs are expressed by adipose tissue and circulate in plasma. Their plasma levels vary with the genetic background, age, gender, and metabolic states. In terms of CTRPs sexually dimorphic patterns, female mice express higher levels of CTRP13, CTRP11, CTRP9, CTRP5, and CTRP3 compared to male animals/humans[1-5]. The exact mechanism for these differences is still unclear.
Figure 1. Structural organization of the CTRPs. A: domain structure of CTRP monomeric protein; B: homotrimeric CTRP protein structure. C: CTRP trimeric proteins form higher-order three-dimensional structures. CTRPs: C1q/tumor necrosis factor-related proteins
CTRPs are expressed and distributed in a variety of ways that may determine their diverse biological functions [Table 1]. To date, metabolic functions have been established for CTRP1, CTRP2, CTRP3, CTRP5, and CTRP9[2]. Although the functional receptors for CTRPs have not yet been recognized, vascular, liver, muscle, heart and adipose tissue are the likely targets of CTRPs. They share similar structure and function with adiponectin[6], including the regulation of balance of body energy metabolism through enhancing insulin sensitivity in the liver and muscle, exhibition of anti-inflammatory responses, and protection of the cardiovascular system (CVS). Thus, the CTRP family may have parallel implications for energy homeostasis and provides new pharmacological targets in the obesity-related diseases and type 2 diabetes. Meanwhile, they consist of many family members distributed in various organs, hence they have the potential to serve as biomarkers in the obesity-related diseases and may serve as detectable parameters for early prognosis and diagnosis.
Overview of the function of CTRP family
| CTRP | Expression | Function |
|---|---|---|
| 1 | Heart, liver, muscle, kidney, sexual gland | Metabolic regulation: glucose and lipid hemostasis; antithrombotic effects; attenuate plaque formation; increases aldosterone production[18,54,55] |
| 2 | Adipose tissue | Metabolic regulation: improve insulin and lipid tolerance[18] |
| 3 (3A,3B) | Kidney, brain, small intestine, adipose tissue | Regulates glucose and lipid metabolism regulates systemic inflammation in obesity and insulin resistance; modulates mitochondrial biogenesis; attenuate liver fibrosis; biomarker for diabetic retinopathy; negative regulator of osteoclastogenesis; independent predictor for atherosclerosis[47,56-60] |
| 4 | Hepatic cells | Modulates energy metabolism; novel nutrient-responsive central regulator of food intake and energy balance; reduces colitis; suppresses the pyroptosis of trophoblasts derived from rats with preeclampsia[61-63] |
| 5 | Adipose tissue, ocular tissue | Inhibits pro-metabolic insulin signaling; related to the degree of obesity and is associated with obesity-related at alterations; associated with ISR after PCI with DES implantation; associated with obesity and diabetes; Alleviates insulin resistance; circulating markers of metabolic disease, diabetes, nonalcoholic fatty liver disease and age-related macular degeneration[64-67] |
| 6 | Adipose tissue, human synoviocytes | Novel metabolic/immune regulator; modulating both inflammation and insulin sensitivity; regulates fat development; alleviates AngII-induced hypertension and vascular endothelial dysfunction; potential therapeutic target for the prevention of skin fibrosis; endogenous complement regulator[5,29,68,69] |
| 7 | Adipose tissue, lung | Improves insulin sensitivity; beneficial metabolic outcomes in the setting of obesity and diabetes[9,54] |
| 8 (8B) | Lung, testis, absent in mice | Blocks glioblastoma dissemination within the brain[14,70] |
| 9 (9A,9B) | Cardiac tissue, adipose tissue | Important in the development of type 2 diabetes; novel metabolic regulator and a new component of the metabolic network that links adipose tissue to lipid metabolism in skeletal muscle and liver; prevents vascular restenosis after angioplasty, hepatic steatosis and hypertension; stabilizes plaque, improves endothelial cell survival and function[12,20,48,71-73] |
| 10 | Eye, adipose tissue | Regulates metabolism, adipose tissue homeostasis[3,23] |
| 11 | Adipose tissue | New regulator of adipogenesis; maintains adipose tissue homeostasis[3,74] |
| 12 | Adipose tissue | Novel biomarkers for the prediction and early diagnosis of T2DM; regulates glucose and lipid metabolism and whole-body glucose homeostasis[24,50,51,75,76] |
| 13 | Adipose tissue, brain | Associated with increased risk of T2DM and coronary artery disease and non-alcoholic fatty liver disease; negative association with metabolism; modulates whole-body energy balance[2,24,27,77,78] |
| 14 | Brain, adipose tissue | Promotes tissue regeneration, and recovery of ischemic heart disease; maintains adipose tissue homeostasis by generating complexes with CTRP11[74] |
| 15 | Skeletal muscle | Modulates energy homeostasis and metabolic circuit; modulates inter-tissue crosstalk[21,79] |
CTRPs in circulation
Endogenous CTRPs can be detected in the blood by enzyme-linked immunosorbent assay (ELISA), even although they circulate at 1-2 orders of magnitude less than adiponectin. The distribution of CTRPs levels varies and is influenced by metabolic hormones, significant signaling and inflammatory states, meanwhile, they can exist in autocrine, paracrine, and endocrine manners that influence the individual susceptibility to the disease and therefore, it provides the possibility that they timely reflect the processing of insulin resistance, obesity, and type 2 diabetes. Accumulated studies reveal that circulating levels of CTRP3, CTRP6, CTRP7, CTRP9, CTRP12, and CTRP15 are reduced in diet-induced diabetes or obese mice or humans[4,5,7-9]. CTRP1 and CTRP5 concentrations are higher in obese and diabetic rodents[10]. The characteristics of CTRPs in various situations are listed [Table 2]. While different CTRP oligomers have distinct distribution properties, the functional significance of CTRPs still remains largely undefined.
C1q/TNF-related proteins traits
| Characteristic | Description |
|---|---|
| Metabolism | Regulation of insulin sensitivity, glucose and lipid metabolism; expression of C1q/TNF-related proteins is various in conditions of obesity and diabetes; promotion of insulin sensitivity, promoting insulin resistance; action is local, action is systemic, exertion of effects via central neuron mechanisms or action on peripheral tissues |
| Muscle and liver | Regulation of AMPK signaling pathway Inflammation; inhibition of monocyte chemoattractant releasing; inhibition of LPS-induced basic and common proinflammatory pathways |
| Obesity and type 2 diabetes | Potent inhibition of LPS-induced systemic inflammation; regulation of TLR9 signaling pathway in chronic inflammation[80], biomarker in reflecting the degree of inflammatory response |
CTRPs perturbations and metabolism
Inter-organ communication in the organism is necessary to maintain the integrated control of metabolism and this crosstalk can be realized by the well-orchestrated secreted hormones. The newly discovered CTRPs family has profound significance aiding better understanding of hormonal control of the energy homeostasis based upon the reports from the research group lead by Wong and other researchers in the last decade[11]. They discovered the contributions of various CTRPs hormones to whole-body glucose and lipid metabolic regulation.
CTRPs can dynamically modulate the response to the alterations in short-term nutritional states or the changes in long-term metabolic status. However, the signaling pathways modulated by CTRPs are frequently disrupted by the metabolic perturbations of excess caloric uptake in obesity- and inflammation-induced disorders. Consequently, the hormone dysregulation leads to broad metabolic disorders such as insulin resistance, diabetes, and obesity. Those disruptions include the alterations of structural organization, and post-translational modifications of CTRPs.
For CTRPs structural organization, it has been reported that all CTRPs form trimers, but accumulating reports reveal that CTRP3, CTRP5, CTRP9, CTRP6, CTRP8, CTRP10, CTRP11, CTRP12, CTRP13 and CTRP15 can further assemble into multimeric complexes mediated by the N-terminal Cysteine residues or with the aid of oxidoreductase. Adiponectin, as an insulin sensitizer, assembles with CTRP9 to form heterotrimers[12], by which CTRP9 and adiponectin share the same receptor to exert their cardiovascular protective function[13]. In addition to forming the homo-oligomers, CTRP6/CTRP1, CTRP7/CTRP2, and CTRP2/adiponectin form heterotrimers generating functionally distinct ligands to provide new framework for the action of this family of secreted glycoproteins in normal and diseases states[11]. CTRP9 has 2 isoforms 9A and 9B, whereas CTRP9B requires physical interaction with CTRP9A and adiponectin for completing its function[14]. CTRP8 in addition to forming homotrimers, forms heteromeric complexes with CTRP1, CTRP9 and CTRP10. It effectively activates GPCR relaxin/insulin like family peptide receptor (RXFP1 receptor) to enhance the motility of the glioblastoma through regulating the activity of cathepsin B[15].
CTRPs, as secreted hormones are subjected to multiple functionally relevant post-translational modifications at their highly conserved residues. CTRP12 is glycosylated on the 39th Asparagine amino acid and the 85th Cysteine modified with oligosaccharides which mediates the assembly of oligomeric structure in human embryonic kidney (HEK) 293T cells. However, the exact modification mechanisms are still under investigation[16]. In addition, the two isoforms of CTRP12 differ from the oligomeric structure and are distinct in the regulation of function. Full length CTRP12 preferentially stimulates the Akt signaling in adipocytes, whereas the globular form activates the mitogen-activated protein (MAP) kinase signaling[16]. CTRP9, as a secreted glycoprotein, can be multiple post-translationally modified in multiple ways in its collagen domain. However, since the CTRP9 globular domain is closely similar to that of adiponectin, the interaction between CTRP9 and adiponectin does not need their collagen domains and is independent of posttranslational modification to activate the downstream signaling pathways[12]. Here, we highlight the characteristics of CTRPs and their isoforms; modification of CTRPs could account for the functional diversity of CTRPs.
The circulating levels of CTRPs tend to fluctuate according to sex, age, and alterations in the metabolic states and are sensitive to different responses in mammals. Reports have been made that CTRP6, a protein with fundamentally different modes of action as opposed to other CTRPs characterized to date, is a negative physiological regulator of glucose metabolism in adipocytes, skeletal muscle and liver to control the systemic energy balance. Expression of CTRP6 is upregulated in leptin deficient animals and increases in diabetic animals[8]. However, other researchers have reported that CTRP6 induces fatty acid oxidation in myocytes via AMPK activation[17]. These studies suggest that the newly discovered CTRPs still need to be investigated further in depth to understand their diverse functions. In addition, other CTRPs functional analysis reveals various roles of CTRPs. CTRP2 exerts fatty acid oxidation and glycogen deposition in myotubes[18]. CTRP1 transcript levels are augmented by rosiglitazone in mice, which enhance the insulin sensitizing action on the skeletal muscle via activation of the AMPK and Akt pathways[19]. CTRP3 can be a secreted plasma hormone and regulates hepatic gluconeogenesis. The metabolic regulatory action of CTRP3 is the downregulation of its plasma concentration which indicates that CTRP3 has a significant role in regulation of the lipid metabolism although the exact mechanism has not yet been established. However, CTRP3 deficiency reduces the liver size which indicates that its target organ appears to be the liver. Additionally, reduced circulating level of CTRP3 alters the inflammatory responses in the obese animals and the short-term daily administration of CTRP3 in diet-induced obese mice has been sufficient to improve the fatty liver phenotype, as evidenced by the suppressed triglyceride content and expression of the triglyceride synthesis genes[1,20]. Hence, CTRP3 shows potent action on the regulation of metabolism. Loss of CTRP5 improves the insulin action states and hepatic steatosis. Deletion of CTRP7 attenuates the obesity-linked glucose intolerance of cytokine expression and circulating levels of cytokine[8]. Overexpression of CTRP9 modestly downregulates the serum glucose and insulin levels. CTRP9 and CTRP15 are considered as cardiokine (the heart-derived proteins are termed cardiokines) due to their high profile in the cardiac tissue and for their protective and preventive actions against cardiovascular injury. Although the CTRP9’s functional receptor has not been thoroughly investigated, cadherin family appears to be a potential candidate[21]. Loss of CTRP12 affects the glucose and lipid metabolism in the obese and insulin-resistant mouse models[22]. CTRP13 regulates the metabolism through AMPK activation and decrease of fatty acid-induced JNK signaling[23]. CTRP15 links skeletal muscle and liver to systemic lipid homeostasis[21]. We summarize current understanding of CTRPs metabolic functions and provide insight into the dynamic regulatory role of CTRP on metabolic balance. Although much has been acknowledged since CTRPs were initially defined, many more questions remain to be addressed.
Of all the CTRPs, CTRP1, CTRP3, CTRP9, CTRP12, CTRP13 have been reported to exhibit positive metabolic regulation and cardiovascular effects in animal models. However, in human studies, circulating levels of CTRP1 are elevated, while CTRP12 displayed a decrease in type-2 diabetes patients. Thus, circulating CTRP1 and CTRP12 can serve as potential novel biomarkers for the early diagnosis of type-2 diabetes in humans[24,25]. The measurement of circulating CTRPs in serum is through commercially available ELISA kits provide by Aviscera company (Santa Clara, USA). CTRP3, in recent human studies, was increased in subjects with the cardio-metabolic syndrome and is associated with various cardio-metabolic risk factors including the triglycerides, high-density lipoprotein-cholesterol, waist-to-hip ratio, and eGFR, which indicate the decreased CTRP3 levels that may serve as a predictor of coronary artery disease, while CTRP13 cannot serve as a predictor candidate since there is evidence that CTRP13 mRNA expression is increased in the setting of obesity[26,27]. This contradiction may be attributed to different nature of human and rodent studies.
Collectively, based upon properties of the known and characterized CTRPS, a strategy designed for screening the biomarkers to predict the metabolic-related disease reveals that CTRP1 and CTRP12 can serve as potential novel biomarkers for the prediction and early diagnosis of type-2 diabetes, and furthermore, they represent a new treatment strategy. CTRP3 can be a better predictor for coronary artery disease than other CTRPs.
CTRPs and inflammation
Metabolic syndrome such as obesity is associated with the chronic low-level inflammation, and therefore the metabolic regulators connecting obesity and diabetes to the inflammatory response have attracted much attention. In addition, it has been gradually recognized that the imbalance of anti-inflammatory adipokines and pro-inflammatory factors leads to the progression of obesity-related diseases. Disrupted anti-inflammatory adipokines participate in the systemic or local inflammatory reactions contributing to the initiation and development of metabolic dysfunction and cardiovascular events. In this regard, to define the imbalance of anti-inflammatory adipokines (CTRPs) and proinflammatory factors (risks factor) would be valuable in studying the obesity-associated complications. Hence, this section will emphasize the possible associations of CTRPs with cardiovascular inflammation.
CTRPs as a priming potential biomarker for predicting the dysfunctional metabolic status and therapeutic target has drawn much attention. Thus, their capability of regulation of metabolism has been widely investigated and documented. However, the association between CTRPs and inflammation, insulin resistance/obesity-linked inflammation, and pro-inflammatory cytokines needs to be thoroughly investigated even although these relationships between those disorders besides CTRPs have been well documented.
CTRPs protect against the complications of obesity such as cardiovascular diseases (CVDs) via their anti-inflammatory properties. Obesity is characterized by chronic inflammation leading to the obesity-related diseases including hypertension, atherosclerosis, and diabetes, while CTRPs as secretory proteins circulating in the organism can easily reach the site of infection to exert its anti-inflammatory characteristics.
Recent studies have shown new insights into CTRP6. For example, upregulation of CTRP6 in the leptin knock-out mice and under diabetic conditions shows distinct roles of CTRP6 in modulating inflammation[5]. In contrast to other CTRPs, CTRP6 expression is obviously elevated in adipose tissue and vascular cells in obese, diabetic patients and mouse models. Overexpression of CTRP6 not only impairs glucose disposal in response to glucose challenge in animal models, but also augments local inflammation by targeting the inflammatory cells in production of TNF-α and IL-10[28]. Circulating inflammatory cytokines and pro-inflammatory macrophages were suppressed in the CTRP6-deficient mouse[5]. In addition, CTRP6 exhibits an immune-regulatory role in the complement system as it specifically suppresses the alternative pathway by binding of the finalizing factor-B to treat arthritis[29], where CTRP6 displays ability to cure the arthritis by intra-articulate injection in mice model[29].
Differing from CTRP6, other CTRPs exhibit the inflammatory regulatory role in a distinct way. In contrast to suppression of CTRP6 expression by cytokines, cytokines IL-1β and TNF-α elevate the expression of CTRP1 adaptively in the adipose tissue by which CTRP1 suppresses inflammation[30]. Meanwhile, CTRP1 impedes collagen-induced platelet coagulation, indicating its potent therapeutic value for treating vascular disorders during the inflammatory process[31]. CTRP3 has been shown to be capable of decreasing the secretion of pro-inflammatory mediators by primary human leukocytes, suggesting strongly an anti-inflammatory function, comparable to adiponectin[32-34]. CTRP5 appears to be a better biomarker than CTRP3 for predicting the severity of obstruction of airflow and systemic inflammation in patients with COPD although concentration alteration in response to the obese women[35,36]. CTRP4 can modulate tumor-promoting inflammation in cancer[37]. CTRP12 has been identified as an anti-inflammatory molecule by Enomoto et al.[38] in 2013. Even among the diverse types of cells, CTRP12 exhibits similar anti-inflammatory actions through the MAPK/JNK- dependent regulatory pathway to modulate TNF-α production.
Various CTRPs exert beneficial actions on the obesity-associated complications including inflammatory disorders through their anti-inflammatory actions. But, numerous functional, physiological, and mechanistic questions regarding the role of CTRPs in inflammation remain to be answered. The establishment and availability of transgenic and knock-out mice models for investigating inflammatory response of CTRPs will advance the knowledge of functions and mechanisms of action of CTRPs. Meanwhile, the receptor(s) responsible for the CTRP-mediated signal transduction should be thoroughly characterized and established.
CTRPs and cardiovascular system
Reports show that CTRPs have beneficial function in the CVS and it is well established that the circulating levels of CTRPs are negatively correlated with the severity of CVDs without or with obesity in animals. Clinic studies indicate close association between CTRPs levels and CVDs. Conversely, the expression of inflammatory mediators in human tissues has been observed to be inversely regulated related to the levels of CTRPs[13,39]. Hence, the reciprocal relationship among CTRPs and the inflammatory mediators may present a dynamic crosstalk in the development of obesity and diabetic complications. Furthermore, the dysregulated production of CTRPs shown in obesity is associated with the pathogenesis of CVDs[40].
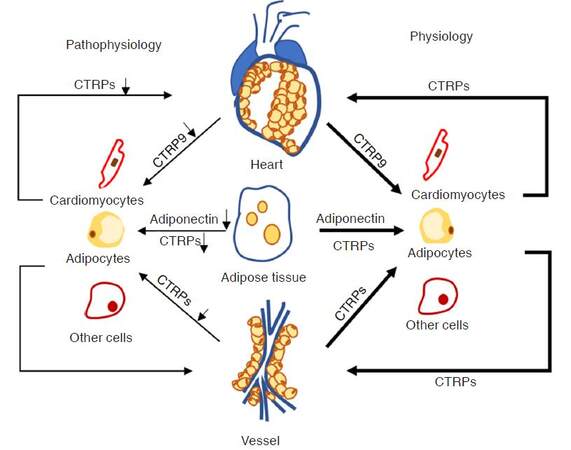
In contrast to adiponectin, which is a well-established adipokine for its anti-inflammatory and insulin sensitizer characteristics, CTRPs show diverse biological activities in the setting of normal metabolic condition and CVDs [Figure 2]. In the past decades, adiponectin has been recognized as a member of the CTRP family as it contains the collagen tail and C1q like globular domain. Recent studies reported that CTRPs exhibit stronger effective and sensitive response than adiponectin to different CVDs. Therefore, in this section, we mainly focus on the characteristics of anti-inflammatory and metabolic regulatory actions of the CTRPs in the onset of metabolic dysfunction-related CVDs.
Figure 2. Pathophysiology of CTRPs in cardiovascular system. CTRPs exerts protective effects in cardiovascular system in physiological condition, but unbalanced levels of CTRPs in pathophysiological condition contributes to the development of cardiovascular relevant disease. CTRPs: C1q/tumor necrosis factor-related proteins
Cardiovascular metabolism plays a pivotal role in the CVDs and the metabolic cascade is particularly important to investigate in the normal and disease processes. Cardiovascular metabolic dysfunction contributes to the progression of obesity-linked cardiovascular events and marks the fate of treatment application. Accumulating studies show that increased levels of CTRP1 are closely associated with the initiation and severity of the coronary arterial disease and can serve as a marker for myocardial infarction[10]. Meanwhile, the inhibition of CTRP1 may slow down the pathogenesis of early stage atherosclerosis and prevent the development of pathological vascular remodeling[41,42]. Circulating levels of CTRP 3 is elevated in patients with dysfunction of glucose metabolism and is associated with numerous cardiovascular metabolic risk factors[26]. It also can serve as a biomarker to predict proliferative retina disorder (PDR) and has beneficial actions for preventing CVDs, providing a promising strategy of vascular remodeling[43]. The levels of CTRP5 are associated with in-stent restenosis after percutaneous coronary intervention (PCI) with drug-eluting-stent implantation. CTRP9 can reflect the pathophysiology of renal involvement and abnormal glucose metabolism besides impairing vasorelaxation in type 2 diabetics[44]. CTRP15, a newly identified myokine, can link skeletal muscle to lipid homeostasis in response to alterations in energy state and predicting changes in the metabolic circuit[21].
Therefore, among the CTRPs, CTRP1 displays the potential to serve as a marker and therapeutic target in the vascular system and CTRP3 exhibits biomarker features in patients with diabetic-related PDR[45]. CTRP5 appears to have a greater potential to serve as a biomarker to predict the risks in PCI following the clinical operations.
CTRPs and cardiovascular inflammation
Inflammation happens in the cardiac tissue and vasculature as an injurious signal in response to the CVDs or risk factors including hypertension, smoking and diabetes. Those situations combined with metabolic dysfunction will amplify the harmful effects to initiate chronic inflammatory reactions resulting in rupture, thrombosis and vulnerable plaque. Basic research and epidemiological clinical studies showed that consistent association between parameters of inflammation and risks of impending cardiovascular event. Emerging advanced techniques can detect locally occurring inflammation by means of screening more reliable and accessible marker systemically, which may provide the best identification of individual at risk for cardiovascular disorders and benefit to prognosis and future treatment.
The alteration in levels of CTRPs is associated with increased risks of cardiovascular event and reflect the progression of CVDs. For better establishment of the relationship between inflammation and CVDs, we will address the recently discovered response of CTRPs to inflammation in the cardiac and vascular systems, respectively to lead the researchers to obtain in depth understanding for the novel strategy for cardiovascular risk.
The levels and actions of circulating CTRPs (CTRP1, CTRP3, CTRP6, CTRP9, CTRP12 and CTRP13) in normal subjects and diabetic patients have been examined in a cross- sectional clinical study which reveals that in chronic inflammatory condition. CTRP1 and CTRP12 show opposite changes in the concentration, while CTRP3 may have a beneficial effect on the prevention of vascular remodeling through anti-inflammatory actions providing a promising strategy for the therapy of vascular disease linked to obesity[42,24]. CTRP6, although it has no effect on collagen or fibroblasts proliferation, does suppress the cell migration induced by inflammatory factors[46]. CTRP9 has been reported to exert positive effects on endothelium-dependent vasorelaxation in aortic vessel ring with the vasorelaxative effects induced via the activation of nitric oxide synthase. Besides that, CTRP9 inhibits the remodeling of vascular wall in the mouse wire-injured model through attenuating vascular smooth muscle proliferation. It is notable that those actions have been mediated by CTRP9 through sharing the adiponectin receptor[47,48]. Those studies highlight the potential protective roles of CTRPs in response to the inflammatory stress.
It has been reported that CTRP3 may act as an inflammatory molecule to improve the post-ischemic cardiac function and cardiac remodeling in animal model via the ability to reduce apoptosis. Whereas, CTRP6 may affect the inflammatory states in the context of obesity by stimulating the activation of p42/44 mitogen-activated protein kinase-dependent pathway[28]. Meanwhile, it can increase the production of anti-inflammatory cytokine IL-10 in monocyte-derived macrophages in human patients. In protecting against the inflammatory response of oxidative stress, CTRP9 can reduce the myocardial ischemia injury-induced superoxide generation to suppress apoptosis and shrink the infarct size[49]. Although CTRP12 has been reported to be lower in an obese rodent model, the systemic administration of CTRP12 inhibits the macrophage pro-inflammatory factor and attenuates the infiltration of cells in the obese mice. Hence, CTRP12 not only modulates the glucose homeostasis, but also leads to the suppression of the inflammatory response of white blood cells[50,51].
Those new investigations and the numerous epidemiological studies indicate that the CTRPs family has the capability to modulate the metabolic and related inflammation to maintain the cardiovascular homeostasis. This sheds light on the biomarker features of CTRPs in the assessment of obesity relevant diseases or as the therapeutic link for the treatment of metabolic dysfunction-associated disorders. Even so, large-scale clinical trials to investigate the effects/actions of CTRPs on cardiovascular events are required for a better understanding of the potential risks and beneficial roles in the prevention of cardiovascular risks.
Limitations
Although investigations on the CTRP receptors are ongoing, much work required to be done to understand them better. Adiponectin receptor1 may in part be involved in the effects of CTRP9 on cardiomyocytes and vascular endothelial cells, but research has been limited on the RNA interference approaches. Solid evidence of the CTRP9 interaction with the adiponectin receptor on the cell surface is lacking. A yeast-based assay system for the progestin and adipoQ receptor family (PAQR) receptor activity reveals that the adiponectin receptor is identified as the category of PAQR1 and PAQR2. Adiponectin is identified as an agonist for PAQR3[52,53]. Since CTRP family members share many characteristics with adiponectin, it has been speculated that the CTRP receptors belong to the PAQR family members or share similarity with the adiponectin receptors.
The number of members in the C1q/TNF-related superfamily keeps growing along with the progression of research. C1q engages a broad range of ligands via its globular domain and modulates cells via the collagen region. The members of this new family are involved in processes as diverse as inflammation, metabolism, energy conversation/expenditure, and beyond, including cell differentiation and proliferation. Therefore, the CTRP family is vastly underestimated and its functions need to be further investigated.
Conclusion
Research on CTRPs has provided insights into their metabolic roles and their characteristics of vascular protection. Although abundant knowledge has been gained since the CTRPs were first discovered, additional questions still need to be answered. CTRPs possess unique and shared functions that are supported by research using the advanced manipulative techniques, such as the genetic mouse models and gene engineering, and by population research on obese and diabetic patients. Future studies will reveal novel insights into the physiological and pathological functions of CTRPs, their metabolic behavior, and possible redundancy of CTRPs in health and disease. Additionally, studying the CTRPs receptors and the downstream signals that transduce the actions of CTRP in the cell are major challenges but the research will provide tremendous insights into drug design and identification of biomarker.
Declarations
Authors’ contributionsStructure design, and finalized the manuscript: Y.J. Wang
Collected data and worked on the revision: W.B. Lau, X.L. Ma
Composed the first draft of the article: J.L. Zhao
Drew figures and collated literatures: J. Liu, R. Guo
Financial support and sponsorshipThis work was supported by the following grants: Shanxi Key Subjects Construction; Innovative Talents of Higher Learning Institutions of Shanxi; American Diabetes Association 1-14-BS-218, 1-17-IBS-297; Natural Science Foundation of China, 81670278, 31322026 (to Y.J. Wang), and 81270185, 81470020 (to J.L. Zhao).
Conflicts of interestThere are no conflicts of interest.
Patient consentNot applicable.
Ethics approvalNot applicable.
Copyright© The Author(s) 2017.
REFERENCES
1. Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 2010;285:39691-701.
2. Byerly MS, Swanson R, Wei Z, Seldin MM, McCulloh PS, Wong GW. A central role for C1q/TNF-related protein 13 (CTRP13) in modulating food intake and body weight. PLoS One 2013;8:e62862.
3. Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/tumor necrosis factor-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis. J Biol Chem 2013;288:10214-29.
4. Wolf RM, Steele KE, Peterson LA, Magnuson TH, Schweitzer MA, Wong GW. Lower circulating C1q/TNF-related protein-3 (CTRP3) levels are associated with obesity: a cross-sectional study. PLoS One 2015;10:e0133955.
5. Lei X, Seldin MM, Little HC, Choy N, Klonisch T, Wong GW. C1q/TNF-related protein 6 (CTRP6) links obesity to adipose tissue inflammation and insulin resistance. J Biol Chem 2017;292:14836-50.
6. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995;270:26746-9.
7. Wolf RM, Steele KE, Peterson LA, Zeng X, Jaffe AE, Schweitzer MA, Magnuson TH, Wong GW. C1q/TNF-related protein-9 (CTRP9) levels are associated with obesity and decrease following weight loss surgery. J Clin Endocrinol Metab 2016;101:2211-7.
8. Lei X, Rodriguez S, Petersen PS, Seldin MM, Bowman CE, Wolfgang MJ, Wong GW. Loss of CTRP5 improves insulin action and hepatic steatosis. Am J Physiol Endocrinol Metab 2016;310:E1036-52.
9. Petersen PS, Lei X, Wolf RM, Rodriguez S, Tan SY, Little HC, Schweitzer MA, Magnuson TH, Steele KE, Wong GW. CTRP7 deletion attenuates obesity-linked glucose intolerance, adipose tissue inflammation, and hepatic stress. Am J Physiol Endocrinol Metab 2017;312:E309-25.
10. Wang H, Wang R, Du D, Li F, Li Y. Serum levels of C1q/TNF-related protein-1 (CTRP-1) are closely associated with coronary artery disease. BMC Cardiovasc Disord 2016;16:92.
11. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 2008;416:161-77.
12. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 2009;23:241-58.
13. Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, Sun Y, Lopez BL, Christopher TA, Peterson JM, Wong GW, Yu S, Yi D, Ma XL. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol 2011;31:2616-23.
14. Peterson JM, Wei Z, Wong GW. CTRP8 and CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF family members. Biochem Biophys Res Commun 2009;388:360-5.
15. Klonisch T, Glogowska A, Thanasupawat T, Burg M, Krcek J, Pitz M, Jaggupilli A, Chelikani P, Wong GW, Hombach-Klonisch S. Structural commonality of C1q TNF-related proteins and their potential to activate relaxin/insulin-like family peptide receptor 1 signalling pathways in cancer cells. Br J Pharmacol 2017;174:1025-33.
16. Wei Z, Lei X, Seldin MM, Wong GW. Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem 2012;287:35804-14.
17. Lee W, Kim MJ, Park EJ, Choi YJ, Park SY. C1qTNF-related protein-6 mediates fatty acid oxidation via the activation of the AMP-activated protein kinase. FEBS Lett 2010;584:968-72.
18. Peterson JM, Seldin MM, Tan SY, Wong GW. CTRP2 overexpression improves insulin and lipid tolerance in diet-induced obese mice. PLoS One 2014;9:e88535.
19. Peterson JM, Aja S, Wei Z, Wong GW. CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J Biol Chem 2012;287:1576-87.
20. Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 2013;305:R522-33.
21. Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 2012;287:11968-80.
22. Tan SY, Little HC, Lei X, Li S, Rodriguez S, Wong GW. Partial deficiency of CTRP12 alters hepatic lipid metabolism. Physiol Genomics 2016;48:936-49.
23. Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem 2011;286:15652-65.
24. Bai B, Ban B, Liu Z, Zhang MM, Tan BK, Chen J. Circulating C1q complement/TNF-related protein (CTRP) 1, CTRP9, CTRP12 and CTRP13 concentrations in type 2 diabetes mellitus: in vivo regulation by glucose. PLoS One 2017;12:e0172271.
25. Chalupova L, Zakovska A, Adamcova K. Development of a novel enzyme-linked immunosorbent assay (ELISA) for measurement of serum CTRP1: a pilot study: measurement of serum CTRP1 in healthy donors and patients with metabolic syndrome. Clin Biochem 2013;46:73-8.
26. Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ, Lee KW, Nam MS, Park YS, Woo JT, Kim YS, Choi DS, Youn BS, Baik SH. C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes 2012;61:2932-6.
27. Fadaei R, Moradi N, Baratchian M, Aghajani H, Malek M, Fazaeli AA, Fallah S. Association of C1q/TNF-related protein-3 (CTRP3) and CTRP13 serum levels with coronary artery disease in subjects with and without type 2 diabetes mellitus. PLoS One 2016;11:e0168773.
28. Kim MJ, Lee W, Park EJ, Park SY. C1qTNF-related protein-6 increases the expression of interleukin-10 in macrophages. Mol Cells 2010;30:59-64.
29. Murayama MA, Kakuta S, Inoue A, Umeda N, Yonezawa T, Maruhashi T, Tateishi K, Ishigame H, Yabe R, Ikeda S, Seno A, Chi HH, Hashiguchi Y, Kurata R, Tada T, Kubo S, Sato N, Liu Y, Hattori M, Saijo S, Matsushita M, Fujita T, Sumida T, Iwakura Y. CTRP6 is an endogenous complement regulator that can effectively treat induced arthritis. Nat Commun 2015;6:8483.
30. Kim KY, Kim HY, Kim JH, Lee CH, Kim DH, Lee YH, Han SH, Lim JS, Cho DH, Lee MS, Yoon S, Kim KI, Yoon DY, Yang Y. Tumor necrosis factor-alpha and interleukin-1beta increases CTRP1 expression in adipose tissue. FEBS Lett 2006;580:3953-60.
31. Lasser G, Guchhait P, Ellsworth JL, Sheppard P, Lewis K, Bishop P, Cruz MA, Lopez JA, Fruebis J. C1qTNF-related protein-1 (CTRP-1): a vascular wall protein that inhibits collagen-induced platelet aggregation by blocking VWF binding to collagen. Blood 2006;107:423-30.
32. Kopp A, Bala M, Weigert J, Buchler C, Neumeier M, Aslanidis C, Scholmerich J, Schaffler A. Effects of the new adiponectin paralogous protein CTRP-3 and of LPS on cytokine release from monocytes of patients with type 2 diabetes mellitus. Cytokine 2010;49:51-7.
33. Hofmann C, Chen N, Obermeier F, Paul G, Buchler C, Kopp A, Falk W, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis 2011;17:2462-71.
34. Schmid A, Kopp A, Hanses F, Karrasch T, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem Biophys Res Commun 2014;452:8-13.
35. Choi HY, Park JW, Lee N, Hwang SY, Cho GJ, Hong HC, Yoo HJ, Hwang TG, Kim SM, Baik SH, Park KS, Youn BS, Choi KM. Effects of a combined aerobic and resistance exercise program on C1q/TNF-related protein-3 (CTRP-3) and CTRP-5 levels. Diabetes Care 2013;36:3321-7.
36. Li D, Wu Y, Tian P, Zhang X, Wang H, Wang T, Ying B, Wang L, Shen Y, Wen F. Adipokine CTRP-5 as a potential novel inflammatory biomarker in chronic obstructive pulmonary disease. Medicine (Baltimore) 2015;94:e1503.
37. Li Q, Wang L, Tan W, Peng Z, Luo Y, Zhang Y, Zhang G, Na D, Jin P, Shi T, Ma D, Wang L. Identification of C1qTNF-related protein 4 as a potential cytokine that stimulates the STAT3 and NF-kappaB pathways and promotes cell survival in human cancer cells. Cancer Lett 2011;308:203-14.
38. Enomoto T, Ohashi K, Shibata R, Kambara T, Uemura Y, Yuasa D, Kataoka Y, Miyabe M, Matsuo K, Joki Y, Hayakawa S, Hiramatsu-Ito M, Ito M, Murohara T, Ouchi N. Transcriptional regulation of an insulin-sensitizing adipokine adipolin/CTRP12 in adipocytes by Kruppel-like factor 15. PLoS One 2013;8:e83183.
39. Qu H, Deng M, Wang H, Wei H, Liu F, Wu J, Deng H. Plasma CTRP-3 concentrations in Chinese patients with obesity and type II diabetes negatively correlate with insulin resistance. J Clin Lipidol 2015;9:289-94.
40. Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord 2014;15:111-23.
41. Liu ZH, Li C, Chen JW, Shen Y, Gao J, Shen WF, Zhang RY, Wang XQ, Lu L. C1q/TNF-related protein 1 promotes endothelial barrier dysfunction under disturbed flow. Biochem Biophys Res Commun 2017;490:580-6.
42. Kanemura N, Shibata R, Ohashi K, Ogawa H, Hiramatsu-Ito M, Enomoto T, Yuasa D, Ito M, Hayakawa S, Otaka N, Murohara T, Ouchi N. C1q/TNF-related protein 1 prevents neointimal formation after arterial injury. Atherosclerosis 2017;257:138-45.
43. Lin S, Ma S, Lu P, Cai W, Chen Y, Sheng J. Effect of CTRP3 on activation of adventitial fibroblasts induced by TGF-beta1 from rat aorta in vitro. Int J Clin Exp Pathol 2014;7:2199-208.
44. Fujita T, Watanabe H, Murata Y, Hemmi S, Yabuki M, Fuke Y, Satomura A, Soma M. Plasma C1q/TNF-related protein 9: a promising biomarker for diabetic renal vascular injury. Minerva Urol Nefrol 2017;69:195-200.
45. Yan Z, Zhao J, Gan L, Zhang Y, Guo R, Cao X, Lau WB, Ma X, Wang Y. CTRP3 is a novel biomarker for diabetic retinopathy and inhibits HGHL-induced VCAM-1 expression in an AMPK-dependent manner. PLoS One 2017;12:e0178253.
46. Lei H, Wu D, Wang JY, Li L, Zhang CL, Feng H, Fu FY, Wu LL. C1q/tumor necrosis factor-related protein-6 attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A pathway and inhibiting myofibroblast differentiation. Basic Res Cardiol 2015;110:35.
47. Yang Y, Li Y, Ma Z, Jiang S, Fan C, Hu W, Wang D, Di S, Sun Y, Yi W. A brief glimpse at CTRP3 and CTRP9 in lipid metabolism and cardiovascular protection. Prog Lipid Res 2016;64:170-7.
48. Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, Ogura Y, Yuasa D, Joki Y, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J 2013;27:25-33.
49. Appari M, Breitbart A, Brandes F, Szaroszyk M, Froese N, Korf-Klingebiel M, Mohammadi MM, Grund A, Scharf GM, Wang H, Zwadlo C, Fraccarollo D, Schrameck U, Nemer M, Wong GW, Katus HA, Wollert KC, Muller OJ, Bauersachs J, Heineke J. C1q-TNF-related protein-9 promotes cardiac hypertrophy and failure. Circ Res 2017;120:66-77.
50. Tan BK, Lewandowski KC, O'Hare JP, Randeva HS. Insulin regulates the novel adipokine adipolin/CTRP12: in vivo and ex vivo effects. J Endocrinol 2014;221:111-9.
51. Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem 2012;287:10301-15.
52. Garitaonandia I, Smith JL, Kupchak BR, Lyons TJ. Adiponectin identified as an agonist for PAQR3/RKTG using a yeast-based assay system. J Recept Signal Transduct Res 2009;29:67-73.
53. Gonzalez-Velazquez W, Gonzalez-Mendez R, Rodriguez-del Valle N. Characterization and ligand identification of a membrane progesterone receptor in fungi: existence of a novel PAQR in Sporothrix schenckii. BMC Microbiol 2012;12:194.
54. Rohrbach S, Aurich AC, Li L, Niemann B. Age-associated loss in adiponectin-activation by caloric restriction: lack of compensation by enhanced inducibility of adiponectin paralogs CTRP2 and CTRP7. Mol Cell Endocrinol 2007;277:26-34.
56. Cheng C, Yu S, Kong R, Yuan Q, Ma Y, Yang W, Cao G, Xie L. CTRP3 attenuates hepatic stellate cell activation through transforming growth factor-beta/Smad signaling pathway. Biomed Pharmacother 2017;89:1387-91.
57. Wolf RM, Lei X, Yang ZC, Nyandjo M, Tan SY, Wong GW. CTRP3 deficiency reduces liver size and alters IL-6 and TGFbeta levels in obese mice. Am J Physiol Endocrinol Metab 2016;310:E332-45.
58. Petersen PS, Wolf RM, Lei X, Peterson JM, Wong GW. Immunomodulatory roles of CTRP3 in endotoxemia and metabolic stress. Physiol Rep 2016;4:e12735.
59. Feng H, Wang JY, Zheng M, Zhang CL, An YM, Li L, Wu LL. CTRP3 promotes energy production by inducing mitochondrial ROS and up-expression of PGC-1alpha in vascular smooth muscle cells. Exp Cell Res 2016;341:177-86.
60. Kim JY, Min JY, Baek JM, Ahn SJ, Jun HY, Yoon KH, Choi MK, Lee MS, Oh J. CTRP3 acts as a negative regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling in vitro and RANKL-induced calvarial bone destruction in vivo. Bone 2015;79:242-51.
61. Luo Y, Wu X, Ma Z, Tan W, Wang L, Na D, Zhang G, Yin A, Huang H, Xia D, Zhang Y, Shi X, Wang L. Expression of the novel adipokine C1qTNF-related protein 4 (CTRP4) suppresses colitis and colitis-associated colorectal cancer in mice. Cell Mol Immunol 2016;13:688-99.
62. Tan WF, Wang LL, Li Q, Luo Y, Na DX, Ma Z, Wang L. Prokaryotic expression and polyclonal antibody preparation of human novel gene CTRP4. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2012;28:614-7. (in Chinese)
63. Duan L, Liu Z, Wang L, Ma B, Fan Y, Xu Y, Guo F. C1q and tumor necrosis factor related protein 4 (CTRP4) suppresses caspase-1/IL-1beta inflammatory pathway in trophoblasts of rat models with preeclampsia. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016;32:1441-5. (in Chinese)
64. Schwartze JT, Landgraf K, Spielau U, Rockstroh D, Loffler D, Kratzsch J, Kiess W, Korner A. Adipocyte C1QTNF5 expression is BMI-dependently related to early adipose tissue dysfunction and systemic CTRP5 serum levels in obese children. Int J Obes (Lond) 2017;41:955-63.
65. Shen Y, Li C, Zhang RY, Zhang Q, Shen WF, Ding FH, Lu L. Association of increased serum CTRP5 levels with in-stent restenosis after coronary drug-eluting stent implantation: CTRP5 promoting inflammation, migration and proliferation in vascular smooth muscle cells. Int J Cardiol 2017;228:129-36.
66. Emamgholipour S, Moradi N, Beigy M, Shabani P, Fadaei R, Poustchi H, Doosti M. The association of circulating levels of complement-C1q TNF-related protein 5 (CTRP5) with nonalcoholic fatty liver disease and type 2 diabetes: a case-control study. Diabetol Metab Syndr 2015;7:108.
67. Chavali VR, Khan NW, Cukras CA, Bartsch DU, Jablonski MM, Ayyagari R. A CTRP5 gene S163R mutation knock-in mouse model for late-onset retinal degeneration. Hum Mol Genet 2011;20:2000-14.
68. Wu W, Zhang J, Zhao C, Sun Y, Pang W, Yang G. CTRP6 regulates porcine adipocyte proliferation and differentiation by the AdipoR1/MAPK signaling pathway. J Agric Food Chem 2017;65:5512-22.
69. Chi L, Hu X, Zhang W, Bai T, Zhang L, Zeng H, Guo R, Zhang Y, Tian H. Adipokine CTRP6 improves PPARgamma activation to alleviate angiotensin II-induced hypertension and vascular endothelial dysfunction in spontaneously hypertensive rats. Biochem Biophys Res Commun 2017;482:727-34.
70. Glogowska A, Kunanuvat U, Stetefeld J, Patel TR, Thanasupawat T, Krcek J, Weber E, Wong GW, Del Bigio MR, Hoang-Vu C, Hombach-Klonisch S, Klonisch T. C1q-tumour necrosis factor-related protein 8 (CTRP8) is a novel interaction partner of relaxin receptor RXFP1 in human brain cancer cells. J Pathol 2013;231:466-79.
71. Jia Y, Luo X, Ji Y, Xie J, Jiang H, Fu M, Li X. Circulating CTRP9 levels are increased in patients with newly diagnosed type 2 diabetes and correlated with insulin resistance. Diabetes Res Clin Pract 2017;131:116-23.
72. Jung TW, Hong HC, Hwang HJ, Yoo HJ, Baik SH, Choi KM. C1q/TNF-related protein 9 (CTRP9) attenuates hepatic steatosis via the autophagy-mediated inhibition of endoplasmic reticulum stress. Mol Cell Endocrinol 2015;417:131-40.
73. Li Y, Geng X, Wang H, Cheng G, Xu S. CTRP9 ameliorates pulmonary arterial hypertension through attenuating inflammation and improving endothelial cell survival and function. J Cardiovasc Pharmacol 2016;67:394-401.
74. Liu F, Tan A, Yang R, Xue Y, Zhang M, Chen L, Xiao L, Yang X, Yu Y. C1ql1/Ctrp14 and C1ql4/Ctrp11 promote angiogenesis of endothelial cells through activation of ERK1/2 signal pathway. Mol Cell Biochem 2017;424:57-67.
75. Mehrdadi P, Kolahdouz Mohammadi R, Alipoor E, Eshraghian MR, Esteghamati A, Hosseinzadeh-Attar MJ. The effect of coenzyme q10 supplementation on circulating levels of novel adipokine adipolin/CTRP12 in overweight and obese patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2017;125:156-62.
76. Enomoto T, Shibata R, Ohashi K, Kambara T, Kataoka Y, Uemura Y, Yuasa D, Murohara T, Ouchi N. Regulation of adipolin/CTRP12 cleavage by obesity. Biochem Biophys Res Commun 2012;428:155-9.
77. Afrookhteh A, Emamgholipour S, Alipoor B, Moradi N, Meshkani R, Nasli-Esfahani E, Rahimipour A, Shanaki M. The circulating levels of complement-C1q/TNF-related protein 13 (CTRP13) in patients with type 2 diabetes and its association with insulin resistance. Clin Lab 2017;63:327-33.
78. Shanaki M, Fadaei R, Moradi N, Emamgholipour S, Poustchi H. The circulating CTRP13 in type 2 diabetes and non-alcoholic fatty liver patients. PLoS One 2016;11:e0168082.
79. Seldin MM, Wong GW. Regulation of tissue crosstalk by skeletal muscle-derived myonectin and other myokines. Adipocyte 2012;1:200-2.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Wang YJ, Zhao JL, Lau WB, Liu J, Guo R, Ma XL. Adipose tissue-derived cytokines, CTRPs as biomarkers and therapeutic targets in metabolism and the cardiovascular system. Vessel Plus 2017;1:202-12. http://dx.doi.org/10.20517/2574-1209.2017.29
AMA Style
Wang YJ, Zhao JL, Lau WB, Liu J, Guo R, Ma XL. Adipose tissue-derived cytokines, CTRPs as biomarkers and therapeutic targets in metabolism and the cardiovascular system. Vessel Plus. 2017; 1: 202-12. http://dx.doi.org/10.20517/2574-1209.2017.29
Chicago/Turabian Style
Wang, Ya-Jing, Jian-Li Zhao, Wayne Bond Lau, Jing Liu, Rui Guo, Xin-Liang Ma. 2017. "Adipose tissue-derived cytokines, CTRPs as biomarkers and therapeutic targets in metabolism and the cardiovascular system" Vessel Plus. 1: 202-12. http://dx.doi.org/10.20517/2574-1209.2017.29
ACS Style
Wang, Y.J.; Zhao J.L.; Lau WB.; Liu J.; Guo R.; Ma X.L. Adipose tissue-derived cytokines, CTRPs as biomarkers and therapeutic targets in metabolism and the cardiovascular system. Vessel Plus. 2017, 1, 202-12. http://dx.doi.org/10.20517/2574-1209.2017.29
About This Article
Copyright
Data & Comments
Data
 Cite This Article 4 clicks
Cite This Article 4 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.