The effect of postoperative malperfusion after surgical treatment of type A acute aortic dissection on early and mid-term survival
Abstract
Aim: To evaluate whether postoperative malperfusion (PM) affected in-hospital and long-term survival in acute type A aortic dissection (AAAD) surgical patients and to identify risk factors for PM.
Methods: Patients who underwent AAAD surgery at a single institution between January 2005 and March 2015 were retrospectively analyzed.
Results: Two-hundred fourteen patients with complete data were identified. At presentation, 119 patients (55.6%) showed preoperative malperfusions: 68 (31.8%) were cerebral, 38 (17.7%) were renal, and 13 (6.1%) were mesenteric. PM was found in 55 patients (25.7%). In-hospital mortality was 47.3% (26/55) vs. 22.6% (36/159) in PM and non-PM patients, respectively (P < 0.0001). Independent predictors for in-hospital mortality included being 75 years or older [odds ratio (OR): 1.1, 95% confidence interval (CI): 1.03-1.13, P < 0.001] and having renal PM (OR: 53.5, 95% CI: 3.97-721.3, P < 0.01). Five-year survival was 78.6 ± 7.8% vs. 93.9 ± 3.4% in PM and non-PM patients, respectively (P < 0.001). Independent predictors for long-term survival were being at least 75 years old (OR: 3.7, 95% CI: 0.9-14.0, P = 0.05) and having renal PM (OR: 28.6, 95% CI: 1.8-462.0, P = 0.01). PM and intimal tears distal to the ascending aorta or the proximal aortic arch were also risk factors.
Conclusion: PM, especially with renal involvement, is associated with in-hospital mortality and reduced long-term survival. AAAD surgeries reduced preoperative malperfusions. Sites of cannulation and interventions requiring circulatory arrest during cardiopulmonary bypass were not predictors of PM.
Keywords
Introduction
Acute type A aortic dissection (AAAD) is a life-threatening condition and one of the most challenging diseases faced by cardiothoracic surgeons. Despite preventative measures including early surgical referrals for patients, preoperative care and improved surgical techniques, in-hospital mortality following surgery remains high, ranging from 10% to 30%.[1,2]
Malperfusion of systemic organs is a complication of aortic dissection caused by branch-vessel involvement. Occurrences can result in dangerous end-organ ischemic dysfunctions, especially when involving the brain. Clinical diagnoses are critical to the development of effective treatment strategies. Proper diagnoses also have important influence on immediate and long-term outcomes of treatment.[3-5]
Malperfusion following either type A or type B acute aortic dissection, is a fairly common complication. It can involve several arterial regions including the cerebral, renal, or mesenteric segments, as well as the upper or lower limbs. In the Stanford Classification Type A Dissection (De Bakey Classification Types I and II) surgical treatment can prevent this complication. In the event of malperfusion, distal aortic percutaneous fenestration can allow blood flow to return to the true lumen. Alternatively, when the distal aortic true lumen is completely obstructed, an endovascular stent can be inserted to recanalize the lower arteries (i.e. the celiac trunk, renal, superior mesenteric and iliac arteries). The International Registry of Acute Aortic Dissection (IRAD) does not include malperfusion as an independent predictor of mortality. Nonetheless, several studies have supported the relevance of this complication, given that it increased in-hospital mortality and adversely affected late survival.[6-9]
The aim of this study was to evaluate the effect of postoperative malperfusion (PM) on in-hospital mortality and long-term survival in patients undergoing surgery for AAAD in a single, high-volume aortic surgery center.
Methods
Between January 2005 and December 2015, 227 patients (mean age 62.5 ± 12.6 years) underwent emergent operations for AAAD. The study was approved by the local Institutional Review Board, which waived the need for patient consent. The preoperative patients’ characteristics are given in Table 1.
Preoperative characteristics, n (%)
| Variable | Overall (n = 214) | non-PM (n = 159) | PM (n = 55) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 62.5 ± 12.6 | 62.3 ± 13.5 | 63.2 ± 10.6 | NS |
| Male gender | 156 (72.9) | 114 | 42 | NS |
| Clinical history | ||||
| Hypertension | 188 (87.9) | 138 | 50 | NS |
| Smoke habit | 69 (32.2) | 49 | 20 | NS |
| BMI (kg/m2) > 30 | 48 (22.4) | 33 | 15 | NS |
| History of CAD | 15 (7.0) | 10 | 5 | NS |
| Diabetes on insulin | 11 (5.1) | 7 | 4 | NS |
| Previous cardiac surgery | 9 (4.2) | 6 | 3 | NS |
| Dialisys-dependent renal failure | 4 (1.8) | 1 | 3 | 0.02 |
| Malperfusion, n (%) | ||||
| Overall malperfusion | 119 (55.6) | |||
| Brain | 68 (31.8) | 47 | 21 | NS |
| Kidney | 38 (17.7) | 26 | 12 | NS |
| Visceral | 13 (6.1) | 8 | 5 | NS |
| Entry tear aortic dissection | ||||
| Ascending aorta | 115 (53.7) | 91 | 24 | NS |
| Aortic arch | 33 (15.4) | 23 | 10 | NS |
| Descending aorta | 8 (3.7) | 3 | 5 | 0.02 |
| Unknown | 58 (27.1) | 42 | 16 | NS |
The diagnosis of malperfusion was based on clinical symptoms and/or imaging evidence, i.e. absence of organ perfusion as determined by computed tomography (CT) scan angiography.
Malperfusion was classified as: cerebral if there was presence of a focal or global stroke leading to brain function deterioration that persisted more than 24 h, or a transient ischemic attack; renal if there was an impairment of renal function (e.g. anuria requiring continuous veno-venous hemofiltration, or a two-fold increase of creatinine serum level); or mesenteric if there was evidence of tense abdominal or intestinal dysfunction, or increased serum levels of liver and/or pancreatic enzymes. The database queries were completely obtained from 214 patients.
Surgical techniques
Prior to operative procedures, patients were monitored with Swan-Ganz pulmonary artery catheters, arterial cannulations to ensure continuous arterial blood pressure measurements (i.e. radial or femoral measurements), and corporeal temperature measurements (TC) during surgery (i.e. rectal, esophageal, or tympanic measurements). Additionally, cerebral monitoring was performed with near-infrared spectroscopy (INVOS® System, Somanetics Corp., Troy, MI, USA) and transcranial Doppler measurements of blood flow velocities in the middle and/or anterior cerebral arteries of the Willis circle.
The heart was accessed through a complete median longitudinal sternotomy in all patients. Arterial access for cardiopulmonary bypass was through either the femoral artery (n = 96), the right axillary artery (n = 96), or direct aortic cannulation (n = 22). Aortic repair was performed in conditions of circulatory arrest and moderate hypothermia (25-28 °C) in 124 (58%) patients.
Cerebral perfusion was given in 118 (55.1%) cases. Fifty-three (24.8%) patients received unilateral selective antegrade perfusion across the right axillary artery in the right common carotid artery. Sixty-five (30.4%) patients received bilateral perfusion using the Kazui technique.[10,11]
Three regions of the aorta (ascending, arch and proximal descending) were investigated in each patient to identify sites of intimal tearing; tears were resected whenever possible. Intimal tearing occurred in the ascending aorta in 115 (53.7%) patients, in the arch in 33 (15.4%) patients, and in the descending aorta in 8 (3.7%) patients. In 58 (27.1%) patients no entry tear was found. Seventy-two patients (33.6%) underwent isolated ascending aortic replacement, 50 (23.4%) had hemiarch resection and 38 (17.8%) had both total arch replacement and ascending aortic replacement. The aortic root was replaced in 43 (20.1%) patients using the modified button Bentall technique. Intraoperative data are given in Table 2.
Surgical variables
| Variable | Overall (n = 214) | non-PM (n = 159) | PM (n = 55) | P value |
|---|---|---|---|---|
| Arterial cannulation, n (%) | ||||
| Right axillary | 96 (44.9) | 69 | 27 | NS |
| Right or left femoral | 96 (44.9) | 72 | 24 | NS |
| Central (into the aorta) | 22 (10.2) | 18 | 4 | NS |
| Brain perfusion, n (%) | ||||
| Monolateral | 53 (24.7) | 38 | 15 | NS |
| Bilateral | 65 (30.4) | 48 | 17 | NS |
| Not performed | 96 (44.9) | 74 | 22 | NS |
| Circulatory arrest, n (%) | 125 (58.4) | 90 | 35 | NS |
| Circulatory arrest temperature (°C), mean ± SD | 27 ± 2 | 26.9 ± 2.5 | 27.1 ± 1.9 | NS |
| Surgical times (min), mean ± SD | ||||
| CPB | 161 ± 82 | 160.9 ± 87.5 | 168.8 ± 67.6 | NS |
| Aortic cross clamp | 91 ± 47 | 92.2 ± 46.4 | 95.2 ± 46.4 | NS |
| Circulatory arrest | 38 ± 31 | 37.6 ± 32.4 | 39.5 ± 30.8 | NS |
| Surgical procedures, n (%) | ||||
| AAR | 72 (33.6) | 53 | 19 | NS |
| AAR + hemiarch replacement | 50 (23.4) | 34 | 16 | NS |
| AAR + arch replacement | 38 (17.8) | 29 | 9 | NS |
| Bentall | 43 (20.1) | 36 | 7 | NS |
| Bentall + hemiarch replacement | 11 (5.1) | 7 | 4 | NS |
Data collection
In-hospital mortality events included both intraoperative and postoperative mortality within 30 days after surgery. Clinical follow-up visits were performed every 12 months in our outpatient control unit; CT-angiography and/or echocardiographic data were collected. For patients living far from this institution who could not participate in regular follow-up visits to the department, clinical status was ascertained by personal interviews with the patients and their cardiologists, including the recording of noninvasive tests.
Statistical analyses
Statistical analyses were performed using StatView 4.5 programming (SAS Institute Inc., Abacus Concepts, Berkeley, CA, USA). Student’s t-test for continuous variables and Chi-squared or Fisher’s exact tests for categorical variables were used. To detect independent predictors of in-hospital mortality and PM logistic regression analyses were performed. Statistical significance by univariate analyses (P < 0.10) were required for entry into the multivariate models. Preoperatively analyzed variables included: age, gender, arterial hypertension, smoking status, body mass index, history of concomitant coronary artery disease, diabetes, chronic kidney disease, previous cardiac surgery, left ventricular dysfunction [e.g. left ventricular ejection fraction (LVEF) < 40%], presence of preoperative malperfusion, and the sites of entry tears. Intraoperatively analyzed variables included: cannulation sites, techniques for obtaining brain perfusions, surgical times, whether there was a need for circulatory arrest, and types of operations (i.e. isolated ascending aorta replacement, Bentall procedure, ascending aortic replacement extended to the hemiarch, or total arch replacement). Overall long-term survival (not including operative mortality) was expressed as a mean of the values plus or minus one standard deviation. Survival analyses were computed using the Kaplan-Meier method; Mantel-Cox log-rank tests were used to compare survival estimates between subgroups. Cox regression models were used to evaluate the influence of the variables on time to death in the entire cohort. A P value less than 0.05 was considered statistically significant.
Results
At presentation, 119 (55.6%) patients exhibited preoperative malperfusion: 68 (31.8%) were cerebral with neurological symptoms, 38 (17.7%) were renal, and 13 (6.1%) were mesenteric. The most relevant clinical characteristics of the cohort, stratified according to the presence or absence of PM, were reported in Table 1.
Intraoperative mortality occurred in 14 (6.5%) patients and in-hospital mortality after surgery occurred in 48 (22.4%) patients. Using univariate analyses, significant risk factors for in-hospital mortality included: being at least 75 years old, having a body mass index of more than 30 kg/m2, having preoperative overall or cerebral malperfusion, having longer cardiopulmonary bypass and aortic clamping times, needing concomitant coronary artery bypass grafting (CABG), having renal PM requiring continuous veno-venous hemofiltration, and have postoperative mechanical ventilation for up to 24 h. The multivariate analyses revealed that being 75 years old or older at the time of surgery (P < 0.001) and having renal PM (P < 0.01) were independent predictors of in-hospital mortality [Table 3].
Predictors of in-hospital mortality
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| P value | OR | 95% CI | P value | |
| Age ≥ 75 years | 0.0078 | 1.1 | 1.0-1.1 | 0.0004 |
| Renal PM | < 0.0001 | 53.5 | 4.0-721.3 | 0.0027 |
| Any preoperative malperfusion syndromes | < 0.0001 | 0.14 | ||
| Preoperative cerebral preoperative malperfusion | 0.0345 | 0.62 | ||
| BMI > 30 | 0.0131 | 0.40 | ||
| CABG | 0.0353 | 0.15 | ||
| CPB time | 0.0008 | 0.12 | ||
| Aortic cross clamp time | 0.0225 | 0.90 | ||
Fifty-five (25.7%) patients showed clinical symptoms and/or imaging evidence of PM. In 42 cases, only 1 organ system was affected, including: the brain in 13 (6.5%) patients, the kidneys in 22 (10.3%) patients, and the viscera in 7 (3.3%) patients. In a subgroup of PM patients with just 1 affected organ system, the mortality rate was 40.5%. In 11 patients, PM occurred in 2 organ systems (5 in the brain and kidneys, 3 in the brain and viscera, 3 in the kidneys and viscera); the mortality rate in this subgroup was 63.6%. PM involving all 3 organ systems occurred in just 2 cases and both patients died. Overall, the in-hospital mortality rate in patients affected by any PM was 47.3% (26/55) vs. 22.6% (36/159) in patients without PM (P < 0.001). Univariate analyses identified that the risk factors for any PM included: having a preoperative LVEF of less than 40% (P < 0.01), having preoperative renal dysfunction (P < 0.05) and having an entry tear distal to the ascending aorta or to the proximal aortic arch (P < 0.05). Multivariate analyses determined that having a LVEF value of 40% or less was the only independent predictor of having a PM (P < 0.05) [Table 4].
Predictors of postoperative malperfusion syndrome
| Variable | Univariate | Multivariate |
|---|---|---|
| P value | P value | |
| Preoperative LVEF < 40% | 0.007 | 0.04 |
| Preoperative dialysis | 0.03 | NS |
| Entry tear distal to the ascending aorta - proximal aortic arch | 0.04 | NS |
Preoperative cerebral malperfusion was an independent predictor of cerebral PM [odds ratio (OR): 2.5, 95% confidence interval (CI): 1.0-6.1, P < 0.05]. Interestingly, a LVEF of less than 40% was only a found to be a significant risk factor for renal PM when using univariate analysis techniques (P < 0.0001). Finally, an entry tear distal to the ascending aorta or to the proximal aortic arch requiring extensive repair and longer surgical time was a risk factor of mesenteric PM (P < 0.05, using univariate analyses).
Follow-up results
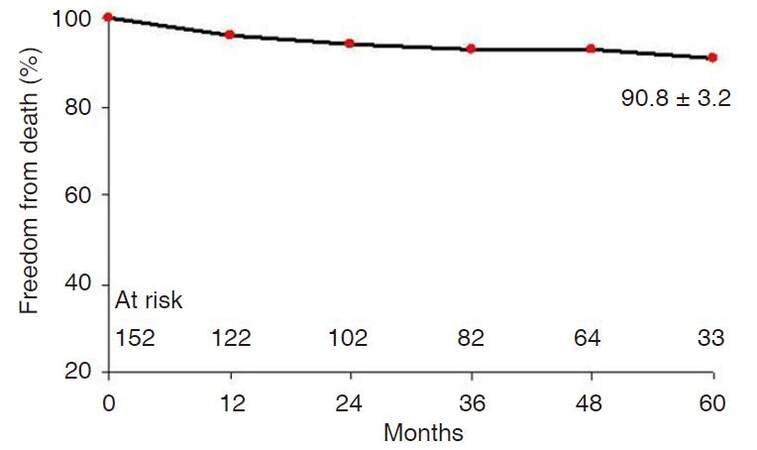
The mean duration of follow-up was 42.4 ± 23.7 months (median 46 months). All patients were followed until the end of the study period. One- and 5-year overall survival rates were 96.0 ± 1.6% and 90.8 ± 3.2%, respectively [Figure 1]. Cox regression analyses identified that independent predictors of long-term survival were: being at least 75 years old at the time of surgery (OR: 3.7, 95% CI: 0.9-14.0, P = 0.05) and having a renal PM (OR: 28.6, 95% CI: 1.8-462.0, P = 0.01) [Table 5]. When the survival probability was dichotomized by age, (with a threshold of 75 years old at the time of the surgery), the 5-year survival rates were 91.6 ± 3.5% for patients < 75 years old and 65.1 ± 19.5% for patients ≥ 75 years old (P < 0.05). The 5-year survival rate for patients without PM was 93.9 ± 3.4% vs. 78.6 ± 7.8% for those affected by PM (Log-rank test, P < 0.01).
Predictors of late mortality
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| P value | OR | 95% CI | P value | |
| Age ≥ 75 years | 0.03 | 3.7 | 0.9-14.0 | 0.05 |
| Renal PM | < 0.0001 | 28.9 | 1.8-462.0 | 0.017 |
| Cerebral PM | 0.001 | NS | ||
| Visceral PM | 0.09 | NS | ||
| Any PM | 0.001 | NS | ||
| Entry tear (distal vs. proximal) of the dissection | 0.02 | NS | ||
Discussion
Despite improvements in medical management and surgical techniques, acute type A aortic dissections still have high mortality and morbidity rates.[1,2] The IRAD revealed that the expected mortality rate for patients undergoing AAAD surgery ranges from 20% to 30%.[9] Our cardiac surgery division has extensive experience in the treatment of acute aortic dissection; we observed an in-hospital mortality rate of 29%. Several studies show that both patient characteristics and multi-organ involvement (i.e. affecting the brain, kidney, and mesenteric organs) play a key role in immediate and post-procedural outcomes. For example, Caus et al.[12] showed that being at least 70 years old at the time of operation was an independent predictor of worsened outcomes for AAAD treated patients; these authors reported a 5-year survival rate of 30%. These data are similar to the clinical experience reported here, in which patients who were at least 75 years old had lower actuarial survival rates than patients under 75 years of age (65.1 ± 19.5% vs. 91.6 ± 3.5%).
Malperfusion of organ systems remains a severe condition that is frequently associated with adverse outcomes in AAAD patients undergoing surgical procedures. Data from the German Registry for AAAD suggested that the number of organs involved in the malperfusion was associated with immediate outcomes of surgery. In fact, outcomes were substantially worsened in the presence of any type of malperfusion syndrome, which was exacerbated with increased numbers of affected organs. A 12.6% early mortality rate was observed in the absence of malperfusion versus 43.4% mortality in patients with three organ systems affected by malperfusion.[13] Here, preoperative clinical symptoms and/or imaging evidence of malperfusion occurred in 119 patients. After surgery, 55 (25.7%) patients had malperfusion syndrome. In-hospital mortality was significantly higher (47.3%, 26 patients) in this group compared to patients without PM (22.6%, 36 patients) (P < 0.0001). Despite the small sample size per group, a strong association between the number of malperfused organs and early mortality was observed. In fact, when 2 organs were affected operative mortality elevated to greater than 60%.
Furthermore, these data show that PM-affected patients had lower survival probabilities when compared to those who did not develop this postoperative complication (78.6 ± 7.8% vs. 93.9 ± 3.4%). Correlations between the preoperative presence of malperfusion and mortality have been previously described.[14,15] Pacini et al.[14] found that patients presenting with any malperfusion syndrome had a mortality rate of 43.7%, compared to 15% in patients without malperfusion (P = 0.001); strikingly, mortality rates were 34.7%, 61.9% and 85.7% with involvement of 1, 2, or more than 2 organ malperfusions, respectively. Mesenteric malperfusion was identified as an independent predictor of operative mortality. Similarly, Geirsson et al.[15] reported a 30.5% operative mortality in the presence of any malperfusion syndrome; in this study cerebral malperfusion was detected as a risk factor for in-hospital mortality (P < 0.001) and reduced long-term survival (P = 0.0002).
In the present study, the most important independent risk factor of early and 5-year mortality was presence of a renal PM requiring continuous veno-venous hemofiltration. Previously, we identified in 100 consecutive patients receiving AAAD operations from 1995 to 2006, that renal failure, either chronic (OR: 0.3, P = 0.04) or developed acutely in the postoperative period (OR: 8.9, P = 0.001), was a predictor of operative mortality. However, renal failure was not a predictor of reduced 5-year survival.[8] In the same group of patients, preoperative LVEF values of less than 50% were also predictors of reduced survival (P = 0.02).
Another important issue is the surgical timing of aortic repairs. Previous authors have suggested delaying acute aortic dissection surgeries when patients experience preoperative malperfusion, particularly in the mesentery. This delayed treatment strategy involved early endovascular treatment with a complete or partial resolution of organ ischemia, followed by timely aortic surgeries.[16,17] While this management approach may be beneficial in a specific subpopulation, patient AAAD survival outcomes have been shown to relate closely to the length of time between diagnoses and surgeries.[18,19] Given the high mortality of patients with mesenteric malperfusion (40-100%), initial management with an interventional procedure treating the condition should be considered.[20,21] In fact, previous data suggested that mesenteric malperfusion was associated with the highest mortality rates when compared to malperfusions occurring in any other organ systems. The surgical strategy presented here, consisting of immediate aortic dissection treatment, showed that incidence of preoperative malperfusion was reduced roughly in half; from 56% preoperatively to 25% in the immediate postoperative period.
Univariate analyses of preoperative variables determined that three risk factors predicted the occurrence of a PM in any organ system. These risk factors were: having a LVEF less than 40%, having renal impairment that required continuous hemofiltration, and having an entry tear distal to the ascending aorta or the proximal aortic arch. However, the only variable that maintained significance in the multivariate model was having a preoperative LVEF of less than 40%. Reduced ejection fraction likely associated with concomitant ischemic coronary disease, which could have increased the risk of a postoperative low cardiac function and subsequent PM. Juxtaposition of intimal tears distal to the ascending aorta or the proximal arch were non-significant factors in the multivariate analyses. However, these factors contributed risk to progression of aortic disease and PM. Patients with a primary entry tear in the descending aorta were at the highest risk of PM. These patients probably required additional extensive repairs compared to patients with primary entry tears in the ascending aorta. Some of these high-risk patients may benefit from a "frozen elephant trunk" procedure to address the entire pathology.[22]
Analysis of preoperative variables contributing risk for each type of PM revealed that only one variable independently predicted cerebral PM: preoperative cerebral malperfusion (OR: 2.5, 95% CI: 1.0-6.1, P < 0.05). Shortening the length of time between onset of cerebral symptoms and dissected aortic surgery was critical for improved outcomes in this subset of patients. Estrera et al.[23] reported improved outcomes in AAAD patients who underwent cardiac surgeries within 10 h of neurological symptom onsets.
With regard to arterial cannulation sites, some authors have suggested that cannulation of the axillary artery will ensure better brain protection during surgery. However, the experience reported here did not confirm this evidence. Nonetheless, many surgeons still limit the extent of surgery to the ascending aorta, even though limited repair has a higher probability of re-intervention on the remaining aortic segments at a later date. The primary aim of performing AAAD is as an emergent, life-saving procedure. If a center is only able to perform a limited repair technique, but still saves the life of the patient, then the primary intention of the procedure has been achieved.[24,25]
In this study, no independent predictors of renal and mesenteric PM were identified. However, using univariate analyses, having a LVEF value less than 40% was statistically relevant (P < 0.0001). Additionally, having entry tears distal to ascending aortic segments that required extensive repairs and longer surgical times was also recognized as a significant risk factor (P < 0.05).
This study had several limitations. First, it was a retrospective analysis of an experience at a single institution. Second, preoperative treatments to address organ malperfusions were not performed. Third, the possible effects of revascularization strategies for the treatment of PM were not explored. Revascularization techniques may improve long-term outcomes.
In conclusion, PM is a severe condition that is frequently associated with adverse immediate and long-term outcomes in surgical AAAD patients. At this institution, the incidence of PM after AAAD surgery was noteworthy, occurring in roughly 10% of patients. AAAD surgical procedures effectively reduced preoperative malperfusions in about half of cases. In fact, repairs to the ascending aorta and proximal arch, as well as removal of primary tears, significantly increased the true lumen flow and allowed treatment of a majority of malperfusion syndromes, including those in the cerebral, mesenteric, and renal systems. Postoperative malperfusion, especially involving the kidneys, was associated with high in-hospital mortality and reduced long-term survival. There was no evidence that the types of surgical techniques undertaken, the sites of cannulation, or the use of more complex interventions (requiring circulatory arrest during cardiopulmonary bypass) were risk factors contributing to PM.
Declarations
Authors’ contributionsStudy design: P. Nardi, C. Olevano, C. Bassano, E. Bovio, G. Ruvolo
Development of methodology: P. Nardi, C. Bassano
Collection of data: C. Olevano, E. Bovio, L. Cecchetti
Analysis and/or interpretation of data: P. Nardi, C. Olevano, C. Bassano, E. Bovio
Writing (not revising) all or sections of the manuscript: P. Nardi, C. Olevano, E. Bovio, S. Forlani
Manuscript review: P. Nardi, E. Bovio, L. Cecchetti
Supervision: G. Ruvolo
Financial support and sponsorshipNone.
Conflicts of interestThere are no conflicts of interest.
Patient consentThe local Institutional Review Board waived the need for patient consent.
Ethics approvalThe study was approved by the local Institutional Review Board.
REFERENCES
1. Colli A, Carrozzini M, Galuppo M, Comisso M, Toto F, Gregori D, Gerosa G. Analysis of early and long-term outcomes of acute type A aortic dissection according to the new international aortic arch surgery study group recommendations. Heart Vessels 2016;31:1616-24.
2. Berretta P, Patel HJ, Gleason TG, Sundt TM, Myrmel T, Desai N, Korach A, Panza A, Bavaria J, Khoynezhad A, Woznicki E, Montgomery D, Isselbacher EM, Di Bartolomeo R, Fattori R, Nienaber CA, Eagle KA, Trimarchi S, Di Eusanio M. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg 2016;5:346-51.
3. Orihashi K. Malperfusion in acute type A aortic dissection: unsolved problem. Ann Thorac Surg 2013;95:1570-6.
4. Fabre O, Guesnier L, Renaut C, Gautier L, Geronimi H, Jasaitis L, Strauch K. Current treatment of acute type A aortic dissection. Surgical treatment and treatment of malperfusion syndrome. Ann Cardiol Angeiol (Paris) 2005;54:332-8. (in French)
5. Immer FF, Grobéty V, Lauten A, Carrel TP. Does malperfusion syndrome affect early and mid-term outcome in patients suffering from acute type A aortic dissection? Interact Cardiovasc Thorac Surg 2006;5:187-90.
6. Zhang J, Jiang Y, Gao C, Feng J, Wang A. Risk factors for hospital death in patients with acute aortic dissection. Heart Lung Circ 2015;24:348-53.
7. Appoo JJ, Pozeg Z. Strategies in the surgical treatment of type A aortic arch dissection. Ann Cardiothorac Surg 2013;2:205-11.
8. Nardi P, Scafuri A, Pellegrino A, Bassano C, Zeitani J, Bertoldo F, Penta de Peppo A, Chiariello L. Surgery for type A aortic dissection: long-term results and risk factor analysis. G Ital Cardiol (Rome) 2007;8:580-5. (in Italian)
9. Rampoldi V, Trimarchi S, Eagle KA, Nienaber CA, Oh JK, Bossone E, Myrmel T, Sangiorgi GM, De Vincentiis C, Cooper JV, Fang J, Smith D, Tsai T, Raghupathy A, Fattori R, Sechtem U, Deeb MG, Sundt TM 3rd, Isselbacher EM; International Registry of Acute Aortic Dissection (IRAD) Investigators. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg 2007;83:55-61.
10. Bassano C, Mve Mvondo C, Bovio E, Chiariello L. Bilateral cerebral perfusion via right axillary artery cannulation alone in aortic arch surgery. Thorac Cardiovasc Surg 2013;61:584-6.
11. Kazui T, Kimura N, Yamada O, Komatsu S. Surgical outcome of aortic arch aneurysms using selective cerebral perfusion. Ann Thorac Surg 1994;57:904-11.
12. Caus T, Frapier JM, Giorgi R, Aymard T, Riberi A, Albat B, Chaptal PA, Mesana T. Clinical outcome after repair of acute type A aortic dissection in patient over 70 years-old. Eur J Cardiothorac Surg 2002;22:211-7.
13. Czerny M, Schoenhoff F, Etz C, Englberger L, Khaladj N, Zierer A, Weigang E, Hoffmann I, Blettner M, Carrel TP. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA registry. J Am Coll Cadiol 2015;65:2628-35.
14. Pacini D, Leone A, Belotti LM, Fortuna D, Gabbieri D, Zussa C, Contini A, Di Bartolomeo R; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg 2013;43:820-6.
15. Geirsson A, Szeto WY, Pochettino A, McGarvey ML, Keane MG, Woo YJ, Augoustides JG, Bavaria JE. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg 2007;32:255-62.
16. Perera N, Galvin SD, Seevanayagam S, Matalanis G. Optimal management of acute type A aortic dissection with mesenteric malperfusion. Interact Cardiovasc Thorac Surg 2014;19:290-4.
17. Yamashiro S, Arakaki R, Kise Y, Inafuku H, Kuniyoshi Y. Management of visceral malperfusion complicated with acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2015;21:346-51.
18. Deeb GM, Williams DM, Bolling SF, Quint LE, Monaghan H, Sievers J, Karavite D, Shea M. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg 1997;64:1669-75.
19. Tsagakis K, Pizanis N, Kamler M, Konorza T, Zoepf T, Erbel R, Jakob H. ICU controlled delay for acute type A aortic dissection repair after intervention for total visceral malpefusion: a way out of a dilemma? Thorac Cardiovasc Surg 2008;56:298-300.
20. Slonim SM, Miller DC, Mitchell RS, Semba CP, Razavi MK, Dake MD. Percutaneous balloon fenestration and stenting for life-threatening ischemic complications in patients with acute aortic dissection. J Thorac Cardiovasc Surg 1999;117:1118-26.
21. Fabre O, Vincentelli A, Willoteux S, Beregi JP, Prat A. Preoperative fenestration for type A acute aortic dissection with mesenteric malperfusion. Ann Thorac Surg 2002;73:950-1.
22. Shrestha M, Fleissner F, Ius F, Koigeldiyev N, Kaufeld T, Beckmann E, Martens A, Haverich A. Total aortic arch replacement with frozen elephant trunk in acute type A aortic dissections: are we pushing the limits too far? Eur J Cardiothorac Surg 2015;47:361-6.
23. Estrera AL, Garami Z, Miller CC, Porat EE, Achouh PE, Dhareshwar J, Meada R, Azizzadeh A, Safi HJ. Acute type A aortic dissection complicated by stroke: can immediate repair be performed safely? J Thorac Cardiovasc Surg 2006;132:1404-8.
24. Wong DR, Coselli JS, Palmero L, Bozinovski J, Carter SA, Murariu D, LeMaire SA. Axillary artery cannulation in surgery for acute or subacute ascending aortic dissections. Ann Thorac Surg 2010;90:731-7.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Nardi P, Olevano C, Bassano C, Bovio E, Cecchetti L, Forlani S, Ruvolo G. The effect of postoperative malperfusion after surgical treatment of type A acute aortic dissection on early and mid-term survival. Vessel Plus 2017;1:77-83. http://dx.doi.org/10.20517/2574-1209.2017.07
AMA Style
Nardi P, Olevano C, Bassano C, Bovio E, Cecchetti L, Forlani S, Ruvolo G. The effect of postoperative malperfusion after surgical treatment of type A acute aortic dissection on early and mid-term survival. Vessel Plus. 2017; 1: 77-83. http://dx.doi.org/10.20517/2574-1209.2017.07
Chicago/Turabian Style
Nardi, Paolo, Carlo Olevano, Carlo Bassano, Emanuele Bovio, Lorenzo Cecchetti, Stefano Forlani, Giovanni Ruvolo. 2017. "The effect of postoperative malperfusion after surgical treatment of type A acute aortic dissection on early and mid-term survival" Vessel Plus. 1: 77-83. http://dx.doi.org/10.20517/2574-1209.2017.07
ACS Style
Nardi, P.; Olevano C.; Bassano C.; Bovio E.; Cecchetti L.; Forlani S.; Ruvolo G. The effect of postoperative malperfusion after surgical treatment of type A acute aortic dissection on early and mid-term survival. Vessel Plus. 2017, 1, 77-83. http://dx.doi.org/10.20517/2574-1209.2017.07
About This Article
Copyright
Data & Comments
Data
 Cite This Article 6 clicks
Cite This Article 6 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.